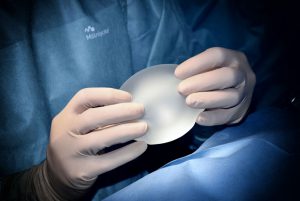
You may have heard more women talking about breast implant illness or chronic symptoms that they have noticed some years after having breast implants. There are certainly now many Facebook groups, online forums, and chats where women share their stories and experiences. With this blog we explore how this may happen, what the symptoms are, and how to navigate safe removal and recovery, if you’re looking to remove implants. You may even like to watch the first ever documentary about breast implant illness by Leigh Wood of South Africa: “Toxic Tits.” Catchy name, hey! 🙂
You may have heard more women talking about breast implant illness or chronic symptoms that they have noticed some years after having breast implants. There are certainly now many Facebook groups, online forums, and chats where women share their stories and experiences. With this blog we explore how this may happen, what the symptoms are, and how to navigate safe removal and recovery, if you’re looking to remove implants. You may even like to watch the first ever documentary about breast implant illness by Leigh Wood of South Africa: “Toxic Tits.” Catchy name, hey! 🙂
Finally after decades of women’s activism, on September 29, 2020 the FDA issued its guidance for their highest level black box warning for breast implants (already categorized as a Class III medical device which comes with the highest warning to users) to further warn users of the negative health affects which many women have experienced.
We have made the links above live so that you can read the FDA guidance which contains recommendations concerning the content and format for certain labelling information for saline and silicone gel-filled breast implants. The FDA issues this guidance to help ensure that a patient receives and understands the benefits and risks of these devices. The recommendations are being made based on concerns that some patients are not receiving and/or understanding information regarding the benefits and risks of breast implants. At the end of this blog, we have also included the Appendix from this FDA document which contains the ingredient list of breast implants.
If you currently have breast implants and are reading this because you have health concerns about them, remember to stay calm and focus on the identification of symptoms and connecting with skilled practitioners and support networks, and remember that the body has an amazing healing and regenerative capacity.
What is Breast Implant Illness?
Breast implant illness is a name that categorises a broad range of symptoms associated with breast implants, which originated in the 1960s. Silicone implants were primarily augmented (the process in which breasts are enlarged through the surgical implantation of false breasts) by women until the early 90s. Saline implants were favoured there-on following the FDA’s acknowledgement and safety concerns of the many health complications associated with silicone implants1.
In the largest study of long-term safety outcomes for patients with breast implants, researchers at The University of Texas MD Anderson Cancer Centre15 have found that silicone implants are associated with some rare diseases, autoimmune disorders, and other conditions, although a causative relationship has not been established. Results of this 2019 study are consistent with previously published studies that examined the safety of breast implants.
The study, published in the Annals of Surgery, is authored by researchers in MD Anderson’s Department of Plastic Surgery and reflects the outcomes of almost 100,000 patients with both saline and silicone implants. The information was derived from the United States Food and Drug Administration’s (FDA) database dating back to 2006, when the United States moratorium on silicone breast implants was lifted. The FDA mandated that breast implant manufacturers perform post-approval studies, primarily to evaluate the potential association of breast implants with rare systemic diseases.
Silicone can migrate from the implant through the body and can induce a chronic inflammatory process and stimulus to the immune system.
Out of 99,993 individuals with silicone implants, there was:
- 6x greater risk of arthritis than the general population
- 4.5x increased risk of having a stillbirth, but not a miscarriage
- 4x greater risk of developing melanoma
According to Mark W. Clemens, M.D., associate professor, Plastic Surgery and senior investigator, the team looked at “certain rare diseases” and found an association with some autoimmune diseases and cancers, including scleroderma and melanoma. “This study did not report a direct link or causative effect between implants and these diseases. It is important to understand a limitation of the study was that some diseases were reported by patients and not necessarily diagnosed by a physician,” said Clemens. He continued by saying, “This is important safety information for women to consider when thinking about cosmetic or reconstructive surgery with breast implants. It also underscores the need for more research in this area,”
According to the study, which reflected data from two implant manufacturers, one group of patients with one brand of silicone implants reported a two to eight-times higher frequency of Sjogren syndrome, rheumatoid arthritis, scleroderma, and melanoma compared to the general population. The other group of patients with another brand of silicone implants used for reconstruction reported scleroderma, Sjogren syndrome and dermatomyositis more than twice as often as the general public. One case of breast implant associated anaplastic large cell lymphoma was reported. “It’s vital that women with implants be aware of the potential risks, so they can identify symptoms early and consult with their doctors,” said Clemens.
Additionally, another study published in October 201816 found an association between having silicone breast implants and a higher likelihood of being diagnosed with various autoimmune/rheumatic disorders such as sarcoidosis, systemic sclerosis and Sjogren’s syndrome. Contrary to manufacturers and plastic surgeon’s telling women that breast implants are safe; the real evidence of breast implant illness and the negative health consequences of breast implants continues to come to light.
This emerging evidence details strong and statistically significant associations of increased autoimmune symptoms and diagnoses, for breast implant women, compared to women without implants, combining data from a wide range of trials and analyses. Concerningly, the study signifies implant patients are more than twice as likely to experience joint stiffness, swelling, and skin and eye dryness.13 Alarmingly, a 90% increase in unspecified rheumatism was noted in women with implants of at least 1 year’s duration. And if that wasn’t enough, a 100% increase for women with breast implants was noted regarding scleroderma, Sjögrens disease, and Rheumatoid Arthritis.14
Mental and cognitive assessment noted a 3-fold increase of fatigue, 6-fold increase for antidepressant use and 6.6-fold increase for the use of sedatives.12 Improved research is needed that focuses on evaluating symptoms and complications of such women, as the FDA is counting surgical procedure numbers, but missing valuable studies regarding the impact on women’s health and wellbeing. Understanding that the research depicted may be increasingly concerning for some, a list of common symptoms associated with the abovementioned conditions is attached below.
Furthermore several reports have shown that fungi (Candida albicans and Aspergillus niger) may be able to survive, colonise, and even cause infection in saline-filled devices.19
Types of Breast Implant Illness:
There are many forms of breast plant illness, most commonly, allergy and autoimmune symptoms appear shortly after augmentation, whilst there are additional long-term dangers related to the procedure. These include cardiovascular symptoms, implant rupture, mental changes, breastfeeding problems, and cancer risk.
The studies outlined above also highlight – swelling, and skin and eye dryness, joint stiffness, unspecified rheumatism, scleroderma, melanoma, sarcoidosis, systemic sclerosis and Sjogren’s syndrome specifically, and an increase in antidepressant and sedative use.13 Breast implants pose many risks, and it doesn’t take long to find these studies and reports, unfortunately to date however the FDA only reports the risk of any adverse reaction to be around 1%.3
 Here’s a summary of some of the main associated symptoms:
Here’s a summary of some of the main associated symptoms:
Chest Pain: Breast implants may aggravate chest pain by provoking chronic inflammation of the lung pleura (the sheet of tissue surrounding your lungs and chest cavity). Symptoms entailing associated chest pain include pain over or around the breasts, stabbing or aching pain in the chest. Implants may also increase your risk of further pulmonary complications due to the chemical reaction initiated to the silicone material of the implants.11
Rupture: It is well documented, that the longer an implant remains in the breast tissue, the greater the chance of rupture. A rupture involves a tearing of the implant where the fluid inside, (most often, silicone) leaks into adjacent tissues, associated with a whole host of additional complications.5 However, please note, implants do not have to rupture to leak silicone into surrounding tissues. Saline implants have valves that may fail and cause leakage of the fluid, whilst silicone can leak from silicone-based implants during needle insertion into the breast tissue, heavy impact, or trauma such as car accidents, or capsular contracture mentioned below.2
Capsular Contracture: Sometimes breast implants may initiate a painful reaction in breast tissue. When the body recognises the breast implant, it builds a fibrous tissue ‘capsule’ around it, mostly made of scar tissue. This process is a protective mechanism of the body in response to a foreign object. This capsule may tighten around the implant over time, contracting and distorting the shape of the breast. Often it can cause the breast to rise higher above the chest and be hard and painful in character.
Unfortunately, rupture and capsular contracture tend to go hand in hand as a previous implant rupture, increases the susceptibility of capsular contracture. Radiation therapy, at any time also raises the risk of contracture, but especially after the initial surgery of augmentation.6
Changes in Brain Function: Fatigue, memory loss, brain fog are symptoms commonly reported after breast implant illness diagnosis and although they are not completely understood by researchers yet, it is important to note that often following removal, these symptoms are reversed.5 It is also likely, negative mental health changes may occur following breast implant illness, especially in instances where women must remove their implants due to complications.
Breastfeeding: Ability to breastfeed following implantation varies from woman to woman, yet importantly, the breast tissue and glands that produce milk may be damaged or completely removed following the implant removal surgery.5
Cancer Risk: A rare form of cancer called Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) has been identified in recent years. This rare cancer is a treatable form of lymphoma that usually resides closely to the scar tissue surrounding the implant. BIA-ALCL often develops after several years following the implant surgery. First signs of the cancer include sudden swelling or hard and painful implants. Additionally, those with textured implants are at greater risk of developing BIA-ALCL, as deemed by the American Society of Plastic Surgeons.4
Treatment of the above symptoms includes removal of the implant and the surrounding tissue and then a detox recovery process.
Here is the FDA’s statement acknowledging breast implant illness and BIA-ALCL cancer caused by breast implants. Have a read of this document because even though the FDA has outlined the risks associated, you are not likely to get help identifying breast implant illness from your doctor or plastic surgeon. There is also no test for breast implant illness, and it appears to be being minimised by plastic surgery associations.
Many women suffering from breast implant illness are misdiagnosed and mistreated for diseases that have similar symptoms and have been repeatedly told their breast implants are not causing their symptoms. Don’t waste time on misdiagnoses and mistreatment. Time is of the essence to your health. Trust your inner wisdom if you feel that your body is reacting to these products, knowing that saline and silicone breast implants made with toxic chemicals and heavy metals do not belong in our healthy bodies.
After explantation (removal) however one study showed that 69% of women experienced a reduction of these symptoms17. While another study found 60-80% of patients showed improved from their signs and symptoms after explantation18. These are significant numbers!
If you do have breast implants, measuring for autoantibodies (for example RF, APF, AKA, AFA, ANA, nDNA, ENA and AAT) would be extremely beneficial to see if your body is reacting and to ensure that your health is not compromised.
Interestingly HLA-DR53 may be a genetic marker of predisposed likelihood of developing disease to silicone implants. Therefore testing for this prior to surgery would be a great option. For some women who have had to have mastectomies, getting breast implants has helped their mental wellbeing enormously. If you’re looking into having breast implants then getting genetically tested prior to the procedure, may be useful in your decision making process one way or the other.
 What’s Involved with Breast Implant Removal?
What’s Involved with Breast Implant Removal?
As many as 20 percent of women who have breast implants need to have their implants removed within eight to 10 years.7 There are multiple ways a breast implant may be removed.
A capsulectomy is a surgical procedure where the implant is removed and replaced by a fresh implant wrapped in a skin substitute made of collagen (termed a dermal matrix). This provides the implant additional protection, allowing the body to renew the scar tissue capsule surrounding the fresh implant.
An open capsulectomy is similar, but involves small incisions to the breast tissue, so the capsule provides more space for the implant to move. Sometimes the implant may be replaced with a fresh one, as with the regular capsulectomy.
During an autologous reconstruction, the implant is removed, and the breast tissue is reconstructed with skin from another part of your body, (usually the buttocks or abdominal area is chosen for its similar texture). This surgery is more complex and longer in recovery yet minimises the risk of capsular contracture reoccurring due to the absence of its formation around the replacement skin.6
Risks and Complications of The Removal?
As with any surgical procedure, the removal of breast implants itself poses risks. Depending on the severity and type of breast implant illness experienced, individuals with breast implants may want to consult their doctor to gather information about the risks of removal, but it is likely if surgery is being considered, that the implants are contributing to symptoms of breast implant illness which need to be addressed.
Bleeding, infection, and scarring are risks commonly attributed to surgical procedures, which require the incision of tissues. These risks are like those outlined before the augmentation in the initial implant procedure.
Ongoing pain may occur even after surgery and may be due to the tissue damage sustained in the surgery itself or associated with a different condition related to the implant illness, so it is always important to monitor your symptoms following the surgery.
Nerve damage is another risk, associated with the removal of breast implants. This may inhibit the sensory function of the nipples, leading to a change or decrease in nipple sensation post-surgery.
As anaesthesia is used during the procedure, complications associated with this may occur, including allergies or nausea.8
All in all, if you are experiencing breast implant illness it is best to have them removed and then work towards restoring your health.
 What Follows Implant Removal?
What Follows Implant Removal?
Detoxing following the removal of breast implants has increased as women have searched for a more natural approach to healing post-surgery. Many steps have been considered, with the most popular ranging from simple dietary and movement changes.
Diet: Supporting the body’s natural systems of detoxification can be obtained through a diet consisting of organic, non-GMO and whole foods. A simple detox diet includes many different vegetables and fruits, those you haven’t heard of, in a variety of colours. Additionally, healthy fats and oils, plentiful amounts of purified, remineralized water will also aid the detoxification process. Removing sugars/sweeteners, dairy, and refined grains, opting for organic ancient grains and natural sources of sweetness e.g., honey, may help to avoid further inflammatory processes in the gut. Avoid processed foods from packaging, especially those containing numbers, colours, or words you do not recognise, and alcohol is of benefit. Incorporate various minerals from clean sources, such as Celtic salt and the consumption of apple cider vinegar or fresh celery juice to allow for an appropriate stomach acidity. It is imperative the body does not have to work harder than in needs to, to detoxify from the food put in it, as it works tirelessly to detoxify from the implant and its removal process.
Heal Your Gut: Many women who have toxicity from breast implants have digestion problems. All inflammatory and allergic foods should be cut out to lower inflammation in the gut and support adequate digestion. Probiotics, especially lactic acid, and soil based, often found in fermented foods are great at healing the healthy gut flora, improving your immune system metabolising toxins from the body.
Exercise: Get exercise each day if possible. Gradually increase intensity to allow proper circulation and lymphatic flow. This enables your body to process the nasties through lymphatic drainage. Breathe fresh air outside, connect with nature, helping to stimulate your rest and digest state where detoxification is possible. Help eliminate inflammation in the body through grounding on the earth each day.
Sauna Therapy and Epsom Salt Baths: Infrared saunas, two to three times each week are a great way to eliminate toxins and heavy metals through the skin by sweating, with the added benefit of infrared light killing microbes within the body. Avoid saunas if you have implants and or mercury fillings. Also take a couple Epsom salt baths a week for magnesium replenishing and detoxification.10
If considering a removal procedure of your breast implants, a list of international surgeons, qualified to perform the surgery is listed here. They can be searched by country and state and contacted directly from this website.9
For further information please visit https://www.healingbreastimplantillness.com
Jennifer Barham-Floreani,
Bach. Chiropractic, Bach. App Clinical Science
Registered internationally, no longer practicing as a chiropractor in Australia.
References:
- Brown, S. L., Langone, J. J., & Brinton, L. A. (1998). Silicone breast implants and autoimmune disease. Journal of the American Medical Women’s Association (1972), 53(1), 21–40.
- Lu, L. B., Shoaib, B. O., & Patten, B. M. (1994). Atypical chest pain syndrome in patients with breast implants. Southern medical journal, 87(10), 978–984. https://doi.org/10.1097/00007611-199410000-00003
- US Food and Drug Administration. 2021
- Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). (2021). Retrieved 31 August 2021, from https://www.plasticsurgery.org/patient-safety/breast-implant-safety/bia-alcl-summary
- Risks of Breast Implants. (2021). Retrieved 31 August 2021, from https://www.fda.gov/medical-devices/breast-implants/risks-and-complications-breast-implants
- Capsular Contracture: Causes, Risks, Signs, Symptoms, and Treatments. (2021). Retrieved 31 August 2021, from https://www.breastcancer.org/treatment/surgery/reconstruction/corrective/capsular-contracture
- Breast augmentation. American Society of Plastic Surgeons. http://www.plasticsurgery.org/cosmetic-procedures/breast-augmentation.html. Accessed July 10, 2018.
- Breast Implant Removal: What to Expect, Pros, Cons, Cost. (2021). Retrieved 31 August 2021, from https://www.healthline.com/health/breast-implant-removal
- EXPLANT SURGEONS ~ Healing Breast Implant Illness. (2021). Retrieved 31 August 2021, from https://www.healingbreastimplantillness.com/explant-surgeons/
- Detox & Heal from Breast Implant Illness – Charlotte Natural Wellness | Functional Naturopathic Medicine | Charlotte, NC. (2021). Retrieved 31 August 2021, from https://charlottenaturalwellness.com/2019/07/02/detox-heal-from-breast-implant-illness/
- Imam H. Shaik, Bindu Gandrapu, Fernando Gonzalez-Ibarra, David Flores, Jyoti Matta, Amer K. Syed, “Silicone Breast Implants: A Rare Cause of Pleural Effusion”, Case Reports in Pulmonology, vol. 2015, Article ID 652918, 3 pages, 2015.https://doi.org/10.1155/2015/65291
- Breiting VB, Holmich LR, Brandt B. “Long-term health status of Danish women with silicone breast implants.” Plastic and Reconstructive Surgery. 2004. https://www.ncbi.nlm.nih.gov/pubmed/15220596
- Laing, T. J., Schottenfeld, D., Lacey, J. V., Jr, Gillespie, B. W., Garabrant, D. H., Cooper, B. C., Heeringa, S. G., Alcser, K. H., & Mayes, M. D. (2001). Potential risk factors for undifferentiated connective tissue disease among women: implanted medical devices.American journal of epidemiology, 154(7), 610–617.
- Aziz NM, Vasey FB, Leaverton PE, et al. “Comparison of clinical status among women retaining or removing gel breast implants.” Presented at the American College of Epidemiology. 1998. https://serials.unibo.it/cgi-ser/start/en/spogli/df- s.tcl?prog_art=632112&language=ENGLISH&view=articoli
- Coroneos CJ, Selber JC, Offodile AC 2nd, Butler CE, Clemens MW. US FDA Breast Implant Postapproval Studies: Long-term Outcomes in 99,993 Patients. Ann Surg. 2019 Jan;269(1):30-36. doi: 10.1097/SLA.0000000000002990. PMID: 30222598.
- Abdulla Watad, Vered Rosenberg, Shmuel Tiosano, Jan Willem Cohen Tervaert, Yarden Yavne, Yehuda Shoenfeld, Varda Shalev, Gabriel Chodick, Howard Amital, Silicone breast implants and the risk of autoimmune/rheumatic disorders: a real-world analysis,International Journal of Epidemiology, Volume 47, Issue 6, December 2018, Pages 1846–1854, https://doi.org/10.1093/ije/dyy217\
- Maijers MC, de Blok CJ, Niessen FB, van der Veldt AA, Ritt MJ, Winters HA, Kramer MH, Nanayakkara PW. Women with silicone breast implants and unexplained systemic symptoms: a descriptive cohort study. Neth J Med. 2013 Dec;71(10):534-40. PMID: 24394743.
- Cohen Tervaert, Jan willem & Colaris, Maartje JL & van der hulst, Rene. (2017). Silicone breast implants and autoimmune rheumatic diseases: myth or reality. Current opinion in rheumatology
- Saray, Aydin & Kilic, Dilek & Kaygusuz, Sedat & Boyunaga, Hakan & Ozlük, Ozlem. (2004). Fungal Growth inside Saline-Filled Implants and the Role of Injection Ports in Fungal Translocation: In Vitro Study. Plastic and reconstructive surgery. 114. 1170-8.
Additional Related Information:
The following Appendix from the FDA’s black box warning on Botox contains certain Labelling Recommendations to Improve Patient Communication regarding their chosen Breast Implants. This document also may be used in Guidance for Industry, and Food and Drug Administration Staff. The said document contains Nonbinding Recommendations.
Appendix C: Materials Device Description Example
The potential toxicity of the chemicals and metals listed in the following tables have been evaluated with both toxicity testing and risk assessments to assess the exposure levels in comparison to the amount determined to likely be safe. However, individual responses to chemicals may vary, and all reactions cannot be predicted.
- Breast Implant Device Materials
| Device Materials | Implant Component |
| Dimethyl Silicone Elastomer Dispersion | Shell |
| Diphenyl Silicone Elastomer Dispersion | Shell |
| MED 4750 Silicone Elastomer | Shell |
| Silicone Gel | Gel fill |
| Platinum catalyst | Shell and fill |
- Chemicals Released by Breast Implants
Volatiles: Chemicals that are released by breast implants as a gas.
Extractables: Chemicals that are released by breast implants following soaking in water and/or organic solvent (liquid).
| Volatiles | Extractables | ||
| Compound | Whole Device (ppm*) | Compound | Whole Device (ppm) |
| D3 Siloxane | 0.18 | D3 Siloxane | 0.5 |
| D4 Siloxane | 0.46 | D4 Siloxane | <2.5 |
| D5 Siloxane | 1.47 | D5 Siloxane | <4.8 |
| Methoxytrimethylsilane | 0.43 | D6 Siloxane | <8.4 |
| Dimethoxydimethylsilane | 0.03 | D7 Siloxane | <8.4 |
| Methoxytriethoxysilane | ND | D8 Siloxane | <8.3 |
| Tetramethyldiethyldisiloxane | 0.04 | D9 Siloxane | <10.92 |
| Acetone | 0.18 | D10 Siloxane | <21.86 |
| Isopropanol | 0.26 | D11 Siloxane | 32.92 |
| 2-Pentanone | ND | D12 Siloxane | 47.85 |
| Methyl Butanoate | 0.01 | D13 Siloxane | 113.11 |
| Ethylbenzene | ND | D14 Siloxane | 172.4 |
| m-& p-xylene | 0.08 | D15 Siloxane | 203.8 |
| 4-Methyl-3-penten-2-one | 0.01 | D16 Siloxane | 584.9 |
| o-xylene | ND | D17 Siloxane | 533.0 |
| Alpha-Pinene | ND | D18 Siloxane | 429.4 |
| Cyclohexanone | ND | D19 Siloxane | 609.9 |
| 1-Ethyl-2-methylbenzene | 0.01 | D20 Siloxane | 775.5 |
| Decane | ND | o-Xylene | <0.4 |
| Benzaldehyde | 0.01 | Siloxane | 3.9 |
| 1,3,5-Trimethylbenzene | 0.01 | Di(Ethylhexyl) Phthalate | ND |
| Limonene | 0.01 | Total Extractables (μg/g) | <4086.7 |
| Undecane | 0.35 | ||
| Acetophenone | 0.01 | ||
| Dodecane | 0.07 | ||
| Total Volatiles | 3.67 | ||
Data preceded with a “<” symbol means that the level of the individual component, if present, was below the method detection limit indicated.
ND=Not detected.
*ppm = parts per million
- Heavy Metals Found in Breast Implants
| Heavy Metals | |
| Metal | Concentration (ppm) |
| Antimony | 0.014 |
| Arsenic | 0.123 |
| Barium | 0.001 |
| Beryllium | 0.006 |
| Cadmium | 0.002 |
| Chromium | 0.028 |
| Cobalt | 0.052 |
| Copper | 0.025 |
| Lead | 0.011 |
| Magnesium | 0.391 |
| Mercury | 0.004 |
| Molybdenum | 0.001 |
| Nickel | 0.050 |
| Platinum | 0.299 |
| Selenium | 0.069 |
| Silver | 0.001 |
| Tin | 0.004 |
| Titanium | 0.033 |
| Vanadium | 0.310 |
| Zinc | 0.034 |