Well that’s a heavy title for a blog! Here’s the thing, “chemicals” tend to get lumped together under the blanket title of “toxins” when in fact sometimes we need to drill down and get specific. This is one of those cases, through understanding the very nature of halogens for example, we better understand how to preserve our health.
Well that’s a heavy title for a blog! Here’s the thing, “chemicals” tend to get lumped together under the blanket title of “toxins” when in fact sometimes we need to drill down and get specific. This is one of those cases, through understanding the very nature of halogens for example, we better understand how to preserve our health.
Here’s the take-home of this blog – right from the start. Iodine deficiency has increased spectacularly because of a lack of iodine in our soil and food, popular diet trends and increased exposure to halogens. There’s not a hormone in the body that’s not reliant on iodine – so, grab a cup of tea and let’s get clear on what we need to know.
So firstly, what’s a halide?
Halogens are a class of elements consisting of fluoride, chlorine, bromine, iodine, and astatine. We briefly discussed their significance in our blog, Iodine – Why You Need It and Why You’ll Love it! please click here to read. Now I’d like to explain how these halogens compete to become “King of the Hill.” Quite literally, inhibiting iodine, and leaving us deficient in this vitally important trace mineral. In this blog we’ll look at how we are exposed to halogens in our modern world and how we can try to avoid or minimise their detrimental effects.
Make sure you stay with me initially here with a brief chemistry refresher and then we’ll move onto how halides are relevant to your health. The halogen family are found in common use in our everyday lives, so it’s important we know how to identify them.
Introducing Halides.
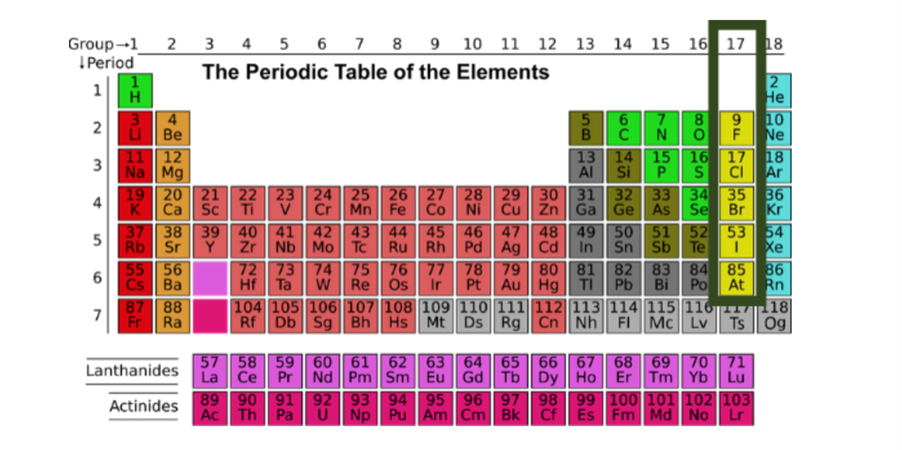
Group VIIA elements on the periodic table include fluorine, chlorine, bromine, iodine, astatine, they are commonly referred to as halogens or halides. Halogen atoms are characterized by an electronic structure where they are missing one electron, so they readily form the anion X2.
Halogens also form complexes like iodide (I3-) that are strongly anionic and oxo-acids and oxides, such as chlorite (ClO2–) and perchlorate (ClO4–) that are also anionic. This all sounds rather nerd worthy and science geeky however it is fairly interesting and relevant, click here for a quick summary on this.
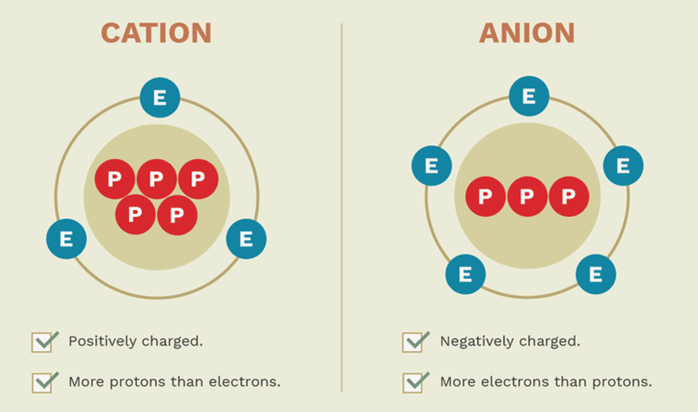
Cations and anions are both ions. The difference between a cation and an anion is the net electrical charge of the ion. Ions are atoms or molecules which have gained or lost one or more valence electrons, giving the ion a net positive or negative charge. If the chemical species has more protons than electrons, it carries a net positive charge. If there are more electrons than protons, the species has a negative charge. The number of neutrons determines the isotope of an element but does not affect the electrical charge.
Cation Versus Anion
Cations are ions with a net positive charge.
Cation Examples:
- Silver: Ag+
- Hydronium: H3O+
- Ammonium: NH4+
Anions are ions with a net negative charge.
Anion Examples:
- Hydroxide anion: OH–
- Oxide anion: O2-
- Sulfate anion: SO42
Because they have opposite electrical charges, cations and anions are attracted to each other. Cations repel other cations and anions repel other anions.
Predicting Cations and Anions
Sometimes, you can predict whether an atom will form a cation, or an anion based on its position on the periodic table. Alkali metals and alkaline earth metals always form cations. Halogens always form anions. Most other nonmetals typically form anions (e.g., oxygen, nitrogen, sulfur), while most metals form cations (e.g., iron, gold, mercury).
According to Wikipedia “… halogens are highly reactive, and as such can be harmful or lethal to living biological organisms in sufficient quantities. This high reactivity is due to the atoms being highly electronegative due to their high effective nuclear charge.”1
The key halogen our body’s need is iodine. In fact, every cell needs iodine, and thyroid and hormonal tissues need it the most. Some people think it is the thyroid gland only, that needs iodine however all the glands in the body need iodine including the ovaries, uterus, breast, prostate, and pancreas. These glands all make hormones. There’s not a hormone in the body that can be produced without iodine.
When we are exposed to halogens other than iodine, they fill the spaces where iodine would normally be found i.e., they are stored in our iodine deficient receptors. It is also known that once these halogens enter the body, they disrupt “ALL” cell enzymes. These cell enzymes cannot then access vital nutritional minerals such as magnesium, zinc, selenium, and iodine.
Let’s pause for a minute.
Now we need a quick refresher on micronutrients so that we better understand iodine. If you haven’t read our first blog on iodine though please click here Iodine – Why You Need It and Why You’ll Love it! please click here to read.
Nutrients are chemical substances required by the body to sustain basic functions and are optimally obtained by eating a balanced diet. There are six major classes of nutrients essential for human health: carbohydrates, lipids, proteins, vitamins, minerals, and water. Carbohydrates, lipids, and proteins are considered macronutrients and serve as a source of energy. Water is required in large amounts but does not yield energy. Vitamins and minerals are considered micronutrients and play essential roles in metabolism. Vitamins are organic micronutrients classified as either water-soluble or fat-soluble. The essential water-soluble vitamins include vitamins B1, B2, B3, B5, B6, B7, B9, B12, and C. The essential fat-soluble vitamins include vitamins A, E, D, and K.
Minerals are inorganic micronutrients. Minerals can classify as macrominerals or microminerals. Macrominerals are required in amounts greater than 100 mg per day and include calcium, phosphorous, magnesium, sodium, potassium, and chloride. Sodium, potassium, and chloride are also electrolytes (a substance that separates in water into ions). Microminerals are those nutrients required in amounts less than 100 mg per day and include iron, copper, zinc, selenium, and iodine.
Iodine is an essential mineral just as iron, magnesium and zinc are – meaning that the body cannot produce them, and without them, we can experience an array of health issues or become seriously ill. Iodine is also known as a trace mineral or micro mineral as the human body only needs these in much smaller amounts, although that doesn’t mean that they are less important.
Now let’s go back to halogens.
As outlined previously, when we are exposed to halogens other than iodine, they fill the spaces where iodine would normally be found i.e., they are stored in our iodine deficient receptors. It is also known that once these toxins enter the body, they disrupt all cell enzymes. These cell enzymes cannot then access vital nutritional minerals such as magnesium, zinc, selenium, and iodine.
This is incredibly important because sometimes we discover that we are deficient in certain minerals and we may even chase our tails getting tested and retested, trying to conquer a nutritional deficiency, when in fact it may be a toxin overload that is at the root cause.
Fascinating – isn’t it?
Now iodine can be bound to amino acids, or it can be free, usually in the form of iodate or iodide ions. Iodide is the easiest form to absorb, so most of the bound iodine and iodate is converted to iodide by glutathione. I tend to bang on a lot about the essential role that glutathione (our most valuable antioxidant), plays in our health so please read our blogs on glutathione starting with Why is Glutathione so Vital?
Iodide is actively transported into the thyroid via the Na+/I−symporter (NIS), a key plasma membrane glycoprotein. This detail becomes important in a moment. Organs with active iodine transport (NaI-symporter system) are primarily the thyroid (about 20 – 40-fold concentration), the stomach, skin, eye (ciliary body), and choroid plexus in the brain. 62
The iodide ions are easily absorbed through the walls of the digestive tract in the stomach and small intestine and after it’s absorbed via the Na+/I−symporter (NIS), most iodine concentrates in the thyroid gland, some of it also accumulates in the ovaries, skin, and salivary, gastric, and mammary glands.62
Stay with me now.
In the case of the halides, which are all antagonistic elements to iodine, they impede the absorption of iodine. Halogens and toxicants, such as perchlorate, nitrate, and thiocyanate, can thereby disrupt normal thyroid function by competitively inhibiting iodide uptake by the sodium/iodide symporter and they are therefore known as NIS-inhibitors.26
Let me put that another way.
The mechanism of iodine in the cells is very ancient and lacking specificity, in fact, cells are not able to distinguish iodide from other anions of similar atomic or molecular size, which may act as “pseudo-iodides”: bromide, fluoride, chlorine, thiocyanate, cyanate, nitrate, pertechnetate, and perchlorate.37 Heavy metals and toxins get stored in the same receptors that are looking for iodine.
As you discover with this blog studies of human NIS indicate that these chemicals act together, to competitively inhibit iodide uptake, i.e., in fact they work cumulatively to inhibit iodine thereby – WORSENING IODINE DEFICIENCY.
This is huge!!
Over the last few decades, we have seen significant increases in thyroid issues from thyroid cancer to autoimmune thyroid disease like Hashimoto’s and Graves’ disease, as well as hypothyroidism, thyroid cancer, pancreatic, ovarian, breast and uterine cancers. More health experts believe that iodine deficiency plays a significant role in these health issues. There are many reasons that an iodine deficiency may occur however over the last few decades it seems that we have become over-chlorinated, over-fluorinated, and over-brominated all the while becoming super deficient in iodine! And NOW we have better insight into why!
To read more about iodine deficiency please read our blog, Iodine – Why You Need It and Why You’ll love it!
Which Halogens Are of Concern?
The elements of the halogen family are found in common use in our everyday life and are harmful to our health. As you now appreciate the key halogen that our body’s need is iodine so let’s look deeply at those halogens of greatest concern – where they are found, and most importantly how we can avoid them. Please click on each of the halogens below for some FASCINATING reading.
Bromide.
Bromide is found naturally in the earth’s crust and seawater. A Bromide ion, Br- releases an electron to form a bromine atom Br. When bromine is added to products it binds to the body’s receptors and blocks the absorption of iodine receptors primarily in the thyroid and stomach.2 Bromine competes for the receptors in the thyroid that capture iodine. In other words, bromine displaces iodine – this problem is called the “Bromide Dominance Theory.” 21Interestingly bromide was used in the past as a sedative to help those suffering from insomnia however, this procedure was stopped when safer alternatives became available.3 Bromine can now be found in several objects around you, including the plastics used to produce computers, fire retardants in fabrics, carpets, and upholstery, and swimming pool treatments. Common sources of bromine also include certain foods and beverages.
Here’s a list of how we are exposed to bromine and bromide:
- Used along with chlorine as a disinfectant and an antibacterial in drinking water to kill bacteria and other potentially harmful microorganisms through a process known as sterilization.
- Used as an antibacterial in hot tubs and pools
- Used in most grains
- Used in bleached and enriched flour. Bromine has been added to flour since the 1980’s as a dough conditioner and may be found in breads and other processed foods. Prior to this iodine was used but bromide proved to be cheaper.
- Used as a flame retardant in furniture, carpets, mattresses, and clothing as Polybrominated diphenyl ethers (PBDEs)
- Used as a preservative in nuts and oils
- Organic bromines are widely used as sprays to kill insects and other unwanted pests on fruits and vegetables. These chemicals are not only poisonous to the pests that they are used against, but also to larger animals but also humans.
- Used as a fumigant for termites
- Used in Paxil and Prozac
- Has been used in carbonated drinks such as Mountain Dew, AMP Energy drink, some Gatorade, Code Red, Dr. Pepper, HC, Sun Drop, Fanta, Minute Maid Lemonade, Citrus Gatorade.
- Used in computers and automobiles
Different Types of Bromide: Next time you are shopping check the labels for the following ingredients:
- Potassium bromates: This is a chemical manufactured in a plant that is added to flours and baked goods to strengthen dough and increase rising height. It is common in processed baked goods. Bread eaters beware!
- Brominated vegetable oil (BVO): This is also a chemical made in a plant, and it is used to help emulsify citrus-flavoured soft drinks, keeping them from separating during distribution i.e., to help keep citrus-flavour oils suspended in beverages and prevent them from floating to the top of the fluid.The status of BVO differs in various parts of the world: It is allowed as a food additive in Latin American and North American countries (including the U.S. and Canada), but not in Japan or European Union countries. See below for further information.
- Methyl bromide: This is a pesticide, and has restricted uses set by the EPA. Even with the restrictions, exemptions are made for strawberry crops, and dry cured pork producers also use it to fumigate their facilities.
- Flame retardants: Commonly used in furniture, electronics, plastics, and even children’s pyjamas as polybrominated diphenyl ethers (PBDEs). These work to slow down chemical reactions that cause a fire.
- Personal care products.: Cetrimonium Bromide is added to certain hair conditions, hair dyes, and other hair products. According to the Skin-Deep website this chemical has a low overall hazard, although allergies and immunotoxicity are possible, and there is a moderate concern of organ system toxicity.
More on Brominated vegetable oil (BVO):
BVO was commonly found in Mountain Dew and other popular citrus-flavoured soft drinks such as Squirt, Fresca, Sunkist Peach, and Fanta Orange, and in sports drinks such as Powerade. In recent years viral online pieces have described BVO as a “toxic chemical” and urged consumers to avoid products such as Mountain Dew soda that contained it4:
“Are you a Mountain Dew addict? Then know what you’re drinking! BVO is a toxic chemical that is banned in many countries because it competes with iodine for receptor sites in the body, which can lead to hypothyroidism, autoimmune disease, and cancer. The main ingredient, bromine, is a poisonous, corrosive chemical, linked to major organ system damage, birth defects, growth problems, schizophrenia, and hearing loss.” There’s flame retardant in your Mountain Dew. That soda with the lime-green hue (and other citrus-flavoured bubbly pops) won’t keep your insides fireproof, but it does contain brominated vegetable oil, a patented flame retardant for plastics that has been banned in foods throughout Europe and in Japan.Brominated vegetable oil, or BVO, which acts as an emulsifier in citrus-flavoured soda drinks, is found in about 10 percent of sodas sold in the U.S.
 “After a few extreme soda binges — not too far from what many gamers regularly consume — a few patients have needed medical attention for skin lesions, memory loss and nerve disorders, all symptoms of overexposure to bromine,” according to a recent article in Environmental News.”
“After a few extreme soda binges — not too far from what many gamers regularly consume — a few patients have needed medical attention for skin lesions, memory loss and nerve disorders, all symptoms of overexposure to bromine,” according to a recent article in Environmental News.”
While food companies stress that the ingredients meet regulatory requirements, their decisions reflect how marketing a product as “natural” has become priority and a competitive advantage. Coca-Cola also said that it’s removing the ingredient from all its drinks to be consistent in the ingredients it uses around the world. Coca-Cola said it would instead use sucrose acetate isobutyrate, which it noted has been used in drinks for more than 14 years, and glycerol ester of rosin, which it said is commonly found in chewing gum and drinks.4 As of June 29, 2020, Mountain Dew’s listed ingredients no longer included BVO.4
Health Conditions Associated with Bromide:
Humans can absorb organic bromines through the skin, with food and during breathing. The problem is that bromide can accumulate in the body and toxicity results in many health issues, including headaches and severe depression. While bromine poisoning, called bromism, is known to cause schizophrenia, delirium, retardation, and hallucinations. In milder cases, it may cause dullness, depression, headaches, and irritability.5 There have been varied cases reported of people consuming high levels of soft drinks and needing to be hospitalised, there has been a particular rise with people “gaming” where players use beverages high in sugar and caffeine to stay alert all night. Sometimes “gamer fuel” includes six large sodas in six hours.
Soda makers and industry groups say they are not concerned about the safety of brominated vegetable oil, saying their products meet all government standards. “This is a safe ingredient approved by the FDA, which is used in some citrus-based beverages,” said Christopher Gindlesperger of the American Beverage Association, which represents PepsiCo, maker of Mountain Dew.6
Some experts are unconvinced, saying that the FDA standards are based on decades-old data. Heather Stapleton, an environmental chemist at Duke University who specializes in studying brominated compounds says that6 “There are some concerns [about BVO] because people are worried that maybe it has the behaviour, and potential health effects similar to brominated flame retardants.”
Charles Vorhees, a toxicologist at Cincinnati Children’s Hospital Medical Center, who studied BVO’s neurological effects in the early 1980s says6,
“Compounds like these that are in widespread use should be re-examined periodically with newer technologies to ensure that there aren’t effects that would have been missed by prior methods, I think BVO is the kind of compound that probably warrants some re-examination. Toxicity testing has changed dramatically in the past few decades. Multiple generations of animals now can be tested for neurodevelopmental, hormonal, and reproductive changes that weren’t imagined in the 1970s and early 1980s. I am no toxicologist, but I think that the toxic evaluation of chemicals has been improved since then.”
- Fatty liver changes, behavioural impairments, and inhibition of reproduction
While the following studies are now old, their results, even without multi-generational results, show compelling evidence of dose-related health effects. In 1970, scientists in England found that rats on a six-week diet containing 0.8 percent brominated maize oil had stockpiles of bromine in their fat tissue. The bromine stayed there even after the rats returned to a control diet for two weeks.6
Around the same time, a study confirmed that bromine was building up in humans. Researchers measured the serum levels of people in the United Kingdom—where BVO was in use—and in their counterparts in the Netherlands and Germany, where BVO was not used.7 “During this time UK citizens had higher bromine serum levels compared to the inhabitants of Germany and the Netherlands,” Vetter said. The largest amounts of lipid-bound bromine were found in tissues from children in the UK, according to the study. The study authors wrote that “it seems highly probable that the intake of brominated vegetable oil is the cause of the tissue bromine residues in children.”
Data in rats show that BVO can be toxic. A 1971 study by Canadian researchers found that rats fed a diet containing 0.5 percent brominated oils grew heavy hearts and developed lesions in their heart muscle. All rats fed the brominated oils also had fatty changes in the liver, but the effect was more marked at 0.5% than at the 0.1% dietary level. Female animals fed the brominated oils had a slightly higher incidence of thyroid microfollicular hyperplasia than males.8
In a later study, in 1983,9 rats fed the same oils had behavioural problems, and those fed 1 percent BVO had severely impaired conception. At this dose postnatal mortality was high, and survivors showed impaired growth and severe behavioural impairments. BVO at 0.5% of the diet produced less reproductive interference but produced behavioural impairments almost as severe as seen in the BVO 1.0% group. BVO at 0.25% of the diet produced reproductive deficits similar to the BVO 0.5% group, but less severe effects on growth and behavioural development. This group showed no significant increase in offspring mortality. While at 2 percent, the rats were unable to reproduce, i.e., the BVO diet completely blocked reproduction.
The data demonstrate clear evidence of dose-related physical and behavioural developmental toxicity.
- Thyroid Issues, Cancer and Cardiovascular disease
With regards to its effects on thyroid health, studies show bromide increases TSH and induces hypothyroidism in rats.10,11 Bromide is also a well-known carcinogen, and there is evidence that it can lead to the development of kidney and thyroid tumours in rats and humans.12 Brominated vegetable oil has also been shown to increase the risk of heart disease.8,13
How To Reduce Your Exposure to Bromide:
- Consume organic foods as often as possible
- Make sure you wash any produce thoroughly with an all-natural fruit and vegetable wash
- Use only organic whole-grain breads and flour
- If you can, grind your own grain
- Be sure to check the labels of commercial baking goods for “no bromine” or “bromine-free” labels
- Avoid sodas and sweetened beverages, and drink only filtered, pure filtered, drinking water
- Do not use plastic containers, as they can contaminate your food, water, and drinks. Instead, use glass containers or ceramic vessels.
Chlorine
Chlorine is another halide, and it is commonly used as a disinfectant. Chloride is an ion of chlorine. It is added to tap water, it’s used in swimming pools, and it’s included in many cleaning products for its antibacterial and whitening properties.
Different types of chlorine:
- Sodium hypochlorite. This is a chemical compound that is also known as bleach (when it’s dissolved in water) and is used in homes and water treatment facilities as a disinfectant.
- Calcium hypochlorite. This is a chemical compound that is granular in texture and is commonly used to disinfect water and is also used in swimming pools as a “shock” but can be a main disinfectant in pools as well.
- Lithium hypochlorite. This is a chemical compound most often used as a disinfectant in swimming pools.
- Cyanuric acidis added to a hypochlorite and is solid in form and used to disinfect outdoor swimming pools. The cyanuric acid stabilizes the chlorine, which means that less of it evaporates due to UV rays.
- Trichlor. This is also a cyanuric acid molecule used to disinfect outdoor swimming pools and is slightly more concentrated than dichlor.
Health Conditions Associated with Chlorine:
Just as is the case with fluoride and bromide, chlorine has been shown to have antithyroid properties, studies demonstrate that it can cause low serum thyroxine (T4) levels14,15,16 and it can also depress T3 levels.17
As you know, chlorination is commonly used for disinfecting swimming pools another chief concern here is the exposure to other toxic compounds, specifically something called disinfection by-products (DBPs). HClO is the active ingredient of chlorination disinfectants, and this is what’s responsible for disinfecting the water. However, HClO is also what produces certain DBPs, which in turn can be inhaled and ingested while swimming. These can also be absorbed through the skin. Two classes of DBPs include chloroform and chloramines. Let’s take a quick look at both:
Chloroform. This is also known as trichloromethane or methyltrichloride. Chloroform is mainly metabolized in the liver, but it can also occur in other tissues of the body, including the kidneys. Chloroform metabolism leads to the production of COCl2, which is highly toxic to cells. According to the EPA, short-term exposure to chloroform through inhalation can cause depression of the central nervous system, while long-term exposure can cause hepatitis, jaundice, and depression.18
Chloramines. These can change into HClO and NH3, which are also toxic to cells. According to the EPA, more than one in five Americans uses drinking water treated with chloramines.19
A meta-analysis that looked at the association between asthma and swimming showed that only competitive swimmers were at risk since they spend much more time in the pool over a period of years.20 This doesn’t mean that there aren’t any risks of swimming in chlorinated pools on an occasional basis.
How To Reduce Your Exposure to Chlorine:
- Avoid drinking tap water. It’s important to consider this when dining out, it’s best to purchase bottled water.
- Invest in a high-quality filter for your drinking water and shower, or get a whole house filter
- Use only natural home-made cleaning products or organic cleaning products.
- Limit time spent in chlorinated swimming pools.
- Consider supplementing withtaurine or glutathione.
Interestingly a study has shown that taurine can help with the excretion of chlorine by increasing the production of intracellular glutathione33. Even though this study specifically mentioned taurine, increasing glutathione levels through other means (i.e., diet, and supplementation) should also help with the excretion of chlorine, along with the other halides discussed. Please read our blogs on glutathione here starting with Why is Glutathione so Vital?
Perchlorate
As we know normal thyroid function is essential for growth and neurological development in foetuses, infants and young children and the thyroid requires iodine to make thyroid hormone. The thyroid gland is the primary target of perchlorate toxicity in humans. The Environmental Protection Agency outlines in their fact sheet that perchlorate can interfere with iodide uptake into the thyroid gland at high enough exposures, disrupting the functions of the thyroid and potentially leading to a reduction in the production of thyroid hormones.34Among women with low urinary iodine concentration <100 μg/l, urinary perchlorate was associated with significant changes in thyroid stimulating hormone and total thyroxine.24,34 Foetuses of pregnant mothers with iodine deficiency are therefore thought to be a sensitive subpopulation for perchlorate exposure25,34 I’ll discuss this more in a moment.
So, what is perchlorate?
Perchlorate can occur naturally, but it is also a man-made type of chemical which is used extensively in the manufacture of flares, fireworks, explosives and especially in rocket fuel. It is also often commonly present in various bleaches, fertilizers, batteries and even airbags. Specifically, perchlorate is a chemical compound containing the perchlorate ion, ClO−4 meaning – it consists of one chlorine atom bonded to four oxygen atoms.
The majority are commercially produced salts mainly used for propellants. Manufactured forms of perchlorate include perchloric acid and salts such as ammonium perchlorate, sodium perchlorate and potassium perchlorate. Perchlorate may occur naturally, particularly in arid regions such as the southwestern United States and is found as a natural impurity in nitrate salts from Chile, which are imported and used to produce nitrate fertilizers, explosives, and other products.36
Human exposure can occur through food or water from natural or industrial sources.22 Reports show an extremely high concentration of perchlorate particulates around airports and that perchlorates have been found to have leached into the ground water supply across most countries from military facilities and the aerospace industry. Highly soluble in water; migrates quickly from soil to groundwater.27
Once dispersed ClO4– can remain in the environment for decades as it does not readily degrade. Perchlorates have also been found in milk, fruit, and vegetables, as well with some studies showing that when lettuce was tested in southern California, 83% of the samples showed significant levels of perchlorate.23
Health Conditions Associated with Perchlorates:
- Thyroid Issues
Interestingly potassium perchlorate was historically used to treat hyperthyroidism because of its ability to inhibit thyroid iodide uptake.36 It makes sense then that exposure to chronic exposure to perchlorate will impact thyroid function.
At high levels, perchlorate can inhibit the function of the thyroid gland, leading to hypothyroidism in adults. Now, a large study published in Environmental Health Perspectives24 shows that much lower levels of perchlorate, traditionally considered safe, can be detrimental to thyroid function in women. The effects in the new study were seen in women who consume less than 100 micrograms of iodine a day, an amount below which, on a population scale, is associated with hypothyroidism, according to the World Health Organization.
The study was conducted by the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia. As part of a regular health survey, researchers measure the levels of some 300 chemicals in a representative sample of people. The study examined the urinary levels of perchlorate and blood levels of thyroxine and thyroid stimulating hormone (TSH) in 2299 men and women aged 12 and older.
Perchlorate had no apparent impact on thyroid hormones in men. For women, higher levels of perchlorate correlated with more TSH. Because iodine deficiency heightens the effect of perchlorate, the team more closely examined those women with less than 100 micrograms iodine per litre of urine. The thinking went that these women might be especially sensitive to perchlorate. For these women, perchlorate was strongly correlated with a small-to-moderate fall in thyroxine and a similar-sized rise in TSH. (When thyroxine is falling, TSH stimulates the thyroid to make more.) “We were not expecting to see the effect,” Pirkle says, because previous studies which tended to combine men and women in the analysis had shown no effects at higher levels of perchlorate.
The Environmental Working Group has done numerous studies25 including the damage caused by perchlorate, these studies demonstrate that they have far reaching effects on women affecting their thyroid and especially pregnant women who even more vulnerable.
- Insulin issues, Diabetes Risk.
Animal studies and now preliminary human studies show that higher urinary perchlorate levels might interfere with insulin secretion and appear to be associated with an increased prevalence of diabetes mellitus, independent of traditional risk factors. Further studies are needed in this area.28
- Learning delays in Children
Several studies have shown that even small changes in T4 and TSH during pregnancy, including those within normal reference ranges, combined with mild maternal iodine deficiency, may be associated with 5–10% decreases in IQ and other important cognitive declines in the offspring.29
Studies demonstrate that perchlorate can be transported across the placental barrier by the sodium-iodide symporter (NIS), resulting in both increased perchlorate exposure and decreased iodide uptake by the foetus.38 In a recent historical cohort study of 21,846 women in Cardiff, United Kingdom, and Turin, Italy,30 who were pregnant from 2002 to 2006, urinary perchlorate levels and subsequent childhood IQ were examined in 487 mother–child pairs in mothers who were hypothyroid (hypothyroxinemic) during pregnancy. This study reported that although associations were not seen with maternal thyroid function, mothers with urine perchlorate concentrations in the highest 10% were at increased risk for having children with IQ scores in the lowest decile at age.
Overall, these studies highlight the potential importance of both perchlorate exposure and small changes in thyroid function during pregnancy.
How To Reduce Your Exposure to Perchlorate:
- Contact your local water supplier to find out if perchlorate is in your drinking water and clarify what steps your utility is taking to reduce your exposure.
Approved laboratories can analyse a sample of your water to determine whether perchlorate is present and at what concentrations.
- Use a high-quality water filter
Perchlorate dissolves easily, is relatively stable and is mobile in water it cannot be removed by heating or boiling water; however, a point-of-use reverse osmosis device could be used to effectively remove perchlorate from drinking water. Regenerable and single-pass ion exchange, reverse osmosis, and fixed- and fluidized-bed biological treatment can all remove perchlorate from drinking water sources.
- Quit smoking
Studies have shown perchlorate accumulates in tobacco plants.36
- Buy organic
While purchasing organic is no guarantee against chemicals found in run-off water, perchlorate has been found in some food crop leaves, and broad-leaf and plants and organic farming methods work to minimise exposure to all environmental chemicals.
- Get your iodine
Since iodine competes with these other halides, we want to make sure that we’re not iodine deficient. Iodine can reduce the effects of perchlorate and keep your thyroid gland healthy. In our blog Iodine, You’ll Love It, we discuss food sources of iodine and recommend taking at least 150 micrograms daily, and 1/2 teaspoon of iodized salt (200 micrograms). Iodine is controversial in the world of thyroid health, and I do not suggest that anyone with a thyroid or autoimmune thyroid condition should supplement with iodine without the guidance of a health practitioner, so be sure to read our related blog before supplementing.
Perchlorates, Nitrates, Thiocyanates, and Iodide Deficiency.
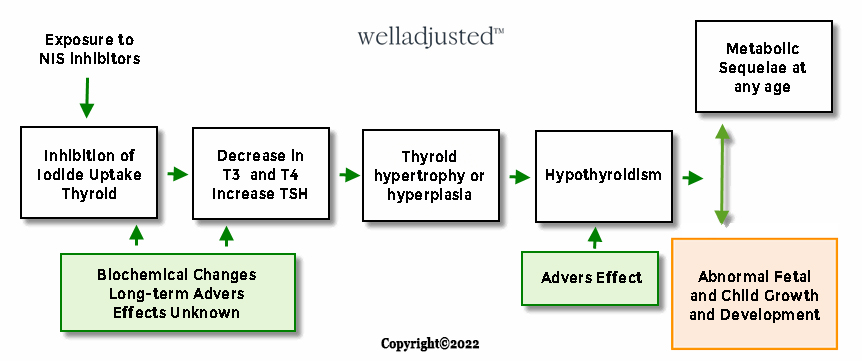
As discussed earlier – halogens and toxicants, such as perchlorate, nitrate and thiocyanate, can disrupt normal thyroid function by competitively inhibiting iodide uptake by the sodium/iodide symporter.31,32 Perchlorate, nitrate and thiocyanate are known as NIS-inhibitors,38 and while nitrate and thiocyanate have lower sodium-iodide symporter (NIS) binding affinities (similarities) than perchlorate, levels of thiocyanate and nitrate in the blood are likely to be higher than levels of perchlorate according to clinical testing34
Studies of human NIS indicate that perchlorate, nitrate, and thiocyanate act additively to competitively inhibit iodide uptake, i.e., as stated earlier in this blog, they work cumulatively to inhibit iodine34 thereby worsening an iodine deficiency by the inhibition of thyroidal iodide accumulation or alternatively by the inhibition of iodide transport into breast milk for infant nutrition. This mechanism must be considered when we consider how low iodine levels impact a developing baby in utero or in childhood.
A large cross-sectional study found increased urinary perchlorate levels were associated with decreased thyroxine and increased thyroid stimulating hormone in women with low urinary iodine with the strongest association being in a subgroup with elevated thiocyanate levels.39 The below diagram helps to explain the above process.
Perchlorate, nitrate, and thiocyanate are ubiquitous in our environment, and exposures can occur through diet (for example, milk, cruciferous, and leafy vegetables) and drinking water. As discussed, perchlorate can occur naturally, but it is also a man-made type of chemical which is used extensively in the manufacture of flares, fireworks, explosives and especially in rocket fuel. It is also often commonly present in various bleaches, fertilizers, batteries and even airbags. Studies have shown perchlorate accumulates in some food crop leaves, tobacco plants and in broad-leaf plants such as cruciferous vegetables.34
Thiocyanate is found in large quantities in foods, particularly cruciferous vegetables, cassava, and certain grains.35 Smoking is a known source of thiocyanate and other goitrogens. In smokers, cyanide from cigarette smoking is likely the most important source of thiocyanate in the body.41 As discussed, perchlorate, nitrate, and thiocyanate are well-known thyroid disrupters and may contribute to changes in body weight. Some studies have found positive associations between urinary thiocyanate with obesity and abdominal obesity.40
Nitrate is commonly found in drinking water as it is added to fertilizers. Specifically, it is commonly found as a co-contaminant in water with perchlorate because ammonium nitrate is a main component in rocket fuel and explosives. Nitrates and nitrites are both naturally occurring chemical compounds found in soil, water, plants, and even our own bodies. One of the most common forms is a natural salt called sodium nitrate, which is exceptionally good at preserving meats and has been used for this purpose for generations. Today a cheaper, chemically altered form of this salt is typically used to preserve small goods. Click here Are Nitrates and Nitrites Good or Bad? for more important health information on nitrates, but with this current blog we are focusing on how nitrate inhibits iodide uptake specifically.
How To Reduce Your Exposure to Thiocyanate:
- Quit smoking
Large amounts of thiocyanate are generated in people with a high intake of cyanide from tobacco smoking.
- Reduce time spent around passive smoke
- Buy organic
Again, while purchasing organic is no guarantee against chemicals found in run-off water, organic farming methods work to minimise exposure to all environmental chemicals.
- Get your iodine
In areas of low iodine intake, thiocyanate exposure increases the risk of developmental and other iodine deficiency disorders. As the overall effect of thiocyanate is to hamper utilization of iodide, the main effect of thiocyanate is to worsen iodine deficiency. By this mechanism thiocyanate is one of the most important environmental compounds influencing the occurrence of thyroid disease.
To read our blog and learn about iodine rich foods and dosages, click here.
How To Reduce Your Exposure to Nitrate:
- Avoid processed meats -ham, bacon, pastrami, salami, hot dogs, and sausages.
If you want to eat processed meats, do so on occasion and look for uncured or nitrate-free labelling. Ham is lower than most processed meats with 0.90 milligrams of nitrtaes per 100 grams of meat. Instead, focus on organic, freshly prepared meats, such as organic beef and chicken or wild caught fish.
- Filter Your Water
Yes, unfortunately nitrates are also found in drinking water. Using a reverse osmosis water filter is probably your safest option.
- Choose Organic
Nitrate is added to fertilizers, which is one way that fruits and vegetables obtain nitrates. As discussed in our blog Are Nitrates and Nitrites Good or Bad? obtaining nitrates from your vegetables should be less of an issue because they contain vitamin C which prevents nitrosamines from forming, however eating plenty of organic fruits and vegetables helps to ensure antioxidant and phytonutrient levels, like vitamin C in your produce. Click here to read.
- Check labels carefully and avoid products that list sodium or potassium nitrates and nitrites.
In addition to lunchmeat, preserved smallgoods, some canned beans and vegetables and even packaged seafood, contain these added chemicals.
- Eat a diet high in antioxidants. Vitamin C and certain other vitamins can reduce the conversion of nitrates and nitrites to nitrosamines.
And finally – Fluoride
Most of us when we think of fluoride, we tend to think of toothpaste. We associate the fluoride that’s added to our toothpaste, mouth rinses, and water supply as a strong disruptor against plaque bacteria helping to prevent tooth decay. Fluoride (from the element fluorine) is a naturally occurring mineral found in the earth’s crust. It is a naturally abundant inorganic mineral found in soil, plants, and bodies of water. Fluoride is created when salts from the element fluorine combine with minerals in soil or rocks. It is found in our freshwater systems and oceans along with animals and plants we eat such as spinach, grapes, and pure chocolate.Once fluoride is absorbed into our body it is stored in our bones and teeth, this process is called remineralization (a process where lost calcium and phosphorous are replenished), in fact, approximately 99% of fluoride found in the body is in our teeth and bones!
Fluoride was discovered as an anti-cavity agent back in the early 1900’s. Interestingly Colorado natives had significant brown staining on their teeth, and their teeth were also surprisingly resistant to cavities. They found out that the source of the staining was the water supply which was rich in fluoride due to natural deposits. We now know this brown staining as fluorosis,42 and it is considered a negative side effect of fluoride today. Eventually local water municipalities began adding fluoride to the water supply to decrease cavities in the public. But it’s important to understand that water municipalities and toothpaste manufacturers aren’t using naturally occurring fluoride. Instead, they’re using the toxic chemical compounds that are by-products of industry. This is where the contention around fluoride begins.
Research studies have shown that fluoride reduces the formation of cavities in both baby and adult teeth, plaque build-up and reduces swelling and bleeding of gums.43
However, for the past 50 to 60 years we have seen the addition of fluoride to our toothpaste, dental floss, mouth rinses, our water supply and some foods and drinks. On any given day – that’s a lot of fluoride. It’s important that we remember that encountering a natural mineral through our diet is entirely different to chronic exposure to a toxic compound.
Fluoride is toxic. In fact, the number one reason for poison control calls concerning fluoride is for children who’ve eaten toothpaste.44
Paul Connett, PhD Co-Founder, Fluoride Action Network says,
“Today, many people living in fluoridated communities are ingesting doses of fluoride (1.6-6.6 mg/day) that fall within the range of doses once used by doctors (2 to 10 mg/day) to reduce thyroid activity in hyperthyroid patients.”45
Let’s read that again.
Symptoms of hypothyroidism are varied and include obesity, lethargy, depression, and heart disease.” Uncontrolled hypothyroidism can raise blood pressure during late pregnancy, increase the risk of miscarriage and preterm delivery, and affect brain development and growth rate. I discuss hypothyroidism further in Iodine, You’ll Love It click here.
Different types of fluoride:
- Calcium Fluoride: Exists naturally in the Earth and is found mostly in limestone. This naturally occurring fluoride is not what’s in toothpaste or added to the water supply.
- Sodium Fluoride: This is a man-made, toxic by-product of the aluminium industry. This was once used as roach and rat poison, it is an ingredient in nerve gas, and it is also used in some anti-depressants42. Sodium fluoride was also used in the past as a treatment for hyperthyroidism due to its anti-thyroid effects, and so this gives you an idea of the negative effect it can have on the body. Sodium fluoride is commonly used as an anti-cavity ingredient in toothpastes & mouthwashes, antidepressants, and it’s also added to the water supply.
- Fluorosilicic Acid: This is a man-made, toxic by-product of fertilizer manufacturing. This is commonly added to the water supply.
- Sodium Hexafluorosilicate: Made by neutralizing fluorosilicic acid with sodium chloride or sodium sulfate. This is also commonly added to the water supply.
Where Is Fluoride Found?
- Fluoride is found in the following dental products:
Toothpaste, cements and fillings, gels and mouthwashes, varnishes, some brands of dental floss, fluoride supplements which are recommended in areas where water is not fluoridated.
- Non-dental sources of fluoride include:
- Water supplies
Water is the major dietary source of fluoride. Lately, major concerns about excessive fluoride intake and related toxicity have led several countries to ban fluoridation. This includes Belgium, Croatia, Finland, France, Germany, Greece, Hungary, Luxembourg, Netherlands, Northern Ireland, Norway, Sweden, Switzerland, Scotland, Iceland, and Italy. In Europe, only Ireland, Poland, Serbia, Spain, and the UK fluoridate their water.
Interestingly Austria has never implemented fluoridation.
Japan only uses fluoridated water through its school programs. Many countries in Central America, Africa, Europe, and the Middle East either have varying accounts, or no information available on the topic of water fluoridation. In the US and Australia about 70-80% of the public water supplies are fluoridated.52
Fluoridated water may be anywhere from 0.7- 1.3 mg/L, often expressed as “ppm” or parts per million. If you live in the typical fluoridated community and you’re drinking your 8 cups of water each day, chances are, you are inadvertently taking in enough fluoride to suppress your thyroid. Most adults living in communities with fluoridated water are ingesting between 1.6 and 6.6 mg of fluoride per day.
Do you live in a fluoridated water community? Be sure to read our blog How to Avoid and Detox from: Fluoride from our fluoride series.
- Fortified foods
Since water fluoridation is not feasible or cost effective in many regions, some countries fortify foods such as milk and salt. Milk contains 2.5 ppm or up to 5 ppm of fluoride usually contains 250 ppm of fluoride51
Fluoridated milk was first investigated in the early 1950s, almost simultaneously in Switzerland, the USA and Japan. Favourable results resulted in a collaboration with the World Health Organization in the early 1980s. Fluoridated milk is often available to children alongside non-fluoridated milk through school milk schemes or national nutritional programmes. At present, milk fluoridation programmes, supported by the WHO and Food and Agriculture Organization, are running continuously in about 15 countries and various channels are used to provide fluoridated milk to children attending kindergarten and school. Currently, more than one and a half million children worldwide consume fluoridated milk however, milk fluoridation is a less efficient method for delivery of fluoride when compared to water fluoridation. The fluoride added to milk forms insoluble complexes that make fluoride absorption difficult.53
Fluoridated salt was initiated in 1955 in Switzerland where 85% of domestic salt consumed is fluoridated and 67% of domestic salt in Germany. France and Austria also have optional salt fluoridation. Salt fluoridation is by far the cheapest method for improving oral health in low socio-economic countries and Salt fluoridation schemes are reaching more than one hundred million in Mexico, Colombia, Peru and Cuba.54
- Drugs containing per fluorinated compounds
They include anaesthetics, antacids, anti-anxiety medications, antibiotics, antidepressants, antifungals, cholesterol-lowering medications, anti-malarial medications, chemotherapy, appetite suppressants, arthritis medications, psychotropics, and steroids. Please click here to read our blog How to Avoid and Detox from Fluoride, for further information on prescription drugs that contain fluoride.
- Food and beverages made with water that contain fluoride.
Black tea, red tea, and other teas, canned food items, black/red rock salt.
Tea leaves accumulate fluoride from the soil, as well as from pollution. The longer they stay on the tea tree, the heavier the fluoride content. (Thus, black tea has more fluoride compared to other teas.) In addition to the tea itself having a high fluoride content, the act of boiling water concentrates the fluoride instead of getting rid of it, contrary to what one might think. On the other hand, freezing the water does not affect the concentration of fluoride. (Opting for white tea, chamomile, and herbal teas, which contain less than 0.13mg/L of fluoride or steeping your black tea for less than one minute, is one way to limit your fluoride exposure.)
- Chewing tobacco
- Pesticides
- Cleaning agents
- Waterproof and stain-resistant items with PFCs
- Teflon, steel, and aluminium products
- Medical imaging scans, such as PET scans
Excess fluoride exposure may also come from:
- High concentrations of fluoride in natural fresh water
Seawater contains 1.2-1.5 ppm of fluoride. Freshwater concentrations are usually lower ranging from 0.01 to 0.3 ppm.
- Untested bottled water
- Inappropriate use of fluoride supplements
- Accidental contamination from fires or explosions
- Large geologic deposits of fluoride, which can contaminate water supplies this is the case for large parts of Africa and Asia
- Volcanic eruptions and rock dissolution
Fluoride Concentrations for Different Types of Food: United States Agriculture Department (USDA 2005).
| Type of Food | Concentration F* (ppm)
1 mg/L = 1ppm |
| Black tea | 3-5 |
| Shellfish products (shrimps, clams) | 2-3 |
| Wine | 1-2 |
| Green tea | 1.2 |
| Chips | 0.7 |
| Beer | 0.5 |
| Boiled or baked pork | 0.42 |
| Boiled rice | 0.41 |
| Salami | 0.4 |
| Bread (with or integral) | 0.39 |
| Cheddar cheese | 0.35 |
| Boiled or raw beef | 0.22 |
| Tuna | 0.2 |
| Chicken meat | 0.15 |
| Plain yogurt | 0.12 |
| Spirits | 0.09 |
| Avocado | 0.07 |
| Boiled pasta | 0.07 |
| Radish | 0.06 |
| Green salad | 0.05 |
Health Conditions Associated with Fluoride:
- Dental fluorosis
Exposure to high concentrations of fluoride during childhood, when teeth are developing, can result in mild dental fluorosis or discolouration of teeth.43 Tiny white streaks or specks in the enamel of the tooth, this apparently does not affect the health of the teeth, but the discoloration may be noticeable. According to the American Dental Association – breastfeeding infants or making up formula milk with fluoride-free water can help protect small children from fluorosis. Fluorosis is one reason that doctors around the world do not recommend that mothers offer their babies water to drink in addition to breast milk or formula. A high-quality water filter however can remedy this. Most formula fed babies become extremely constipated and making additional water available is important from this regard.
The American Dental Association also recommends that children below the age of 6 years should not use a mouthwash that contains fluoride and children should be supervised when brushing their teeth to ensure they do not swallow toothpaste.43
- Hyperparathyroidism (this is different to hyperthyroidism)
In some cases, excess fluoride can damage the parathyroid gland.46,47 This can result in hyperparathyroidism, which involves uncontrolled secretion of parathyroid hormones. This can result in a depletion of calcium in bone structures and higher-than-normal concentrations of calcium in the blood. Lower calcium concentrations in bones make them more susceptible to fractures.48
- Skeletal Fluorosis and Arthritis
Our liver struggles to process fluoride, thus it passes into the bloodstream where it combines with calcium that’s been leeched from the skeletal system. This process leaves our bones weak, otherwise known as skeletal fluorosis. Bones and joints become hardened and less elastic causing pain and increasing the chance of fractures.49 If the bones thicken and bone tissue accumulates, this can contribute to impaired joint mobility.45 Degenerative osteoarthritis has also been linked to skeletal fluorosis.55
- Thyroid problems
As we’ve discussed Iodine and fluoride both belong to the family of halogens. Clinical pharmacist and author Dr Izabella Wentz says that,
“Just like other halogens, fluoride has been proven to act as a trigger in inducing thyroid cell death, as well as lead to the development of thyroid inflammation and autoimmune thyroid disease like Hashimoto’s.”56
Fluoride is toxic to thyroid cells; it inhibits function and causes cell death.57
For decades, fluoride was used to reduce thyroid function in individuals suffering from an overactive thyroid.58 Studies suggest that fluoride mimics the hormone TSH (thyroid stimulating hormone) and disrupts the enzymes that are needed to form the T3 and T4 hormones. In one case-control study 198 cases and 213 controls were selected. Fluoride was determined by the SPADNS Colorimetric Method. It was found that fluoride has impacts on TSH, T3 hormones even in the standard concentration of less than 0.5 mg/L. Fluoridated water may be anywhere from 0.7- 1.3 mg/L, often expressed as “ppm” or parts per million. Application of a high-quality standard household water purification devices was recommended for hypothyroidism. 59
A 2015 British study reported that medical practices in a fluoridated area of the UK (West Midlands vs. those in a non-fluoridated area, Greater Manchester) were TWICE as likely to report a high prevalence rate of hypothyroidism in their patients. Furthermore, an analysis of different parts of the UK found that the rates of hypothyroidism were statistically matched to the rates of fluoride in the local water supply.60 The article reported the following average rates of fluoride in fluoridated communities.
These levels may have since increased:
-
- England 1.0 mg/L
- Canada 0.7 mg/L
- Southern Ireland 0.7 mg/L
- United States 0.7 – 1.3 mg/L
[One litre of water is equivalent to 33.8 ounces, or roughly 4.2 American cups.]
- Neurotoxic effects and lowered IQ
Fluoride was shown to accumulate in rat brain tissues after chronic exposures to high levels, and investigators have speculated that accumulation in the hippocampus might explain the neurotoxic effects on learning and memory61. An experimental study on mice has shown that fluoride exposure may have adverse effects on neurodevelopment, manifesting as both cognitive and behavioural abnormalities later in life.63.
Fluoride has also been shown to delay neurobehavioral development in children64 In 2014, fluoride was documented as a neurotoxin that could be hazardous to child development, along with 10 other industrial chemicals, including lead, arsenic, toluene, and methylmercury.65
In 2017, a report was published suggesting that exposure to fluoride before birth could lead to poorer cognitive outcomes in the future. The researchers measured fluoride levels in 299 women during pregnancy and in their children between the ages of 6 and 12 years. They tested cognitive ability at the ages of 4 years and between 6 and 12 years. Higher levels of fluoride were associated with lower scores on IQ tests.66
A 20 year systematic review in China found that children who live in a fluorosis area have five times higher odds of developing low IQ than those who live in a nonfluorosis area or a slight fluorosis area.67
A 2019 study found that mothers who had consumed fluoridated tap water while they were pregnant tended to give birth to children who ended up having slightly lower IQ scores by ages 3 to 4. Children were born between 2008 and 2012; 41% lived in communities supplied with fluoridated municipal water. The study sample included 601 mother-child pairs recruited from 6 major cities in Canada; children were between ages 3 and 4 years at testing. Data were analysed between March 2017 and January 2019 and children’s IQ was assessed at ages 3 to 4 years using the Wechsler Primary and Preschool Scale of Intelligence-III. In this study, maternal exposure to higher levels of fluoride during pregnancy was associated with lower IQ scores in children aged 3 to 4 years.68
What happens when children are exposed to high fluoride and low iodine?
You guessed it – the impact is far greater. Wechsler Intelligence Test IQ scores of 160 children, 8–14 years old, from nine schools in an area of high fluoride and low iodine averaged 64.8 compared with 85.0 (p<0.01) for 169 children of the same ages from seven schools in an area with low iodine only. Among the first group 65 (40.6%) had IQs below 60, but only 23 (13.6%) among the second group had scores this low. In each group the IQs of the boys and girls did not differ significantly. Clearly, exposure to the combination of high fluoride and low iodine was more deleterious than to low iodine alone.69
Animal studies demonstrated that brain protein was decreased by low iodine and even more by the combined interaction of high fluoride and low iodine. The activity of cholinesterase (ChE) in the brain was affected to some extent by high fluoride and low iodine but was especially affected by high fluoride and low iodine together.70
- Harmful to Male and Female Fertility
A direct link exists between fertility rates and fluoridated drinking water. Higher levels of fluoride correspond to lower fertility rates, particularly with drinking water levels of 3 ppm.71 Animal models show that fluoride reduces reproductive hormones in females.72 While males suffering from fluorosis have lower testosterone and fertility than men with limited fluoride exposure.
- Accelerates Female Puberty
It also deserves mention that the pineal gland plays an integral role in the onset of puberty. Research has shown that girls living in areas prone to more fluoride exposure experience puberty earlier than girls exposed to less.73
Calcifies the Pineal Gland.
The pineal gland regulates body rhythms and wake-sleep cycles; two extremely important functions. Fluoride is especially toxic to the pineal gland, where it accumulates and calcifies the gland. In fact, by the time the average person reaches old age, their pineal gland will have higher calcium density than their bones.74
Research suggests that the presence of fluoride in the pineal gland inhibits enzymes that are essential to produce melatonin (a neurohormone produced by the pineal glands in the brain, mainly at night.)
How To Reduce Your Exposure to Fluoride:
As fluoride is added to tap water, the amount we are exposed to varies from person to person, and this of course depends on how much tap water a person drinks, bathes in, brushes their teeth with, etc. The opportunity for chronic fluoride overexposure is most certainly a cause for concern.
- Avoid drinking tap water
- Don’t cook with tap water
- Avoid drinking tap water. It’s important to consider this when dining out, it’s best to purchase a high-quality bottled water.
- Invest in a high-quality filter for your shower, or get a whole house filter
- Use only fluoride-free toothpastes and mouthwashes
- Avoid fluorinated drugs
- Avoid fluoridated salt and milk
- Avoid fluoride rich foods and drinks
- Get your iodine
I discuss fluoride in depth in a series of three blogs, click here to read.
In conclusion.
The key halogen our body’s need is iodine. As discussed, cells are not able to distinguish iodide from other anions of similar atomic or molecular size, which may act as “pseudo-iodides”: bromide, fluoride, chlorine, thiocyanate, cyanate, nitrate, pertechnetate, and perchlorate blocking iodine and resulting in an iodine deficiency. As you can now appreciate it is incredibly important that we learn how to avoid or minimise our exposure to these halides to both live a healthier lifestyle and support our endocrine system. This is not just important for pregnant women and children; it is integral for all men and women.
Yours in health,
Jennifer Barham-Floreani,
Bach. Chiropractic, Bach. App Clinical Science
Registered internationally, no longer practicing as a chiropractor in Australia.
References:
1.
Wikipedia (2022). Halide. Wikipedia [Sourced Jan. 2022]
https://en.wikipedia.org/wiki/Halide
2
Allain P, Berre S, Krari N, et al (1993). Bromine and thyroid hormone activity. J Clin Pathol. 1993;46(5):456-458.
Osansky, E (2018). Fluoride, Bromide, Chloride and Thyroid Health. Natural Endocrine Solutions. January 2 2018. [Sourced 18/01/22] https://www.naturalendocrinesolutions.com/articles/fluoride-bromide-chloride-and-thyroid-health/
3.
López-Muñoz F, Ucha-Udabe R, Alamo C (2005). The history of barbiturates a century after their clinical introduction. Neuropsychiatr Dis Treat. 2005;1(4):329-343.
Papich, MG (2016). Bromide. Saunders Handbook of Veterinary Drugs (Fourth Edition).
4.
Snopes Staff (2003). Does Mountain Dew Contain the ‘Dangerous Chemical’ BVO? Published 8 March 2013, Updated 2 July 2020. https://www.snopes.com/fact-check/mountain-dew-contain-chemical-known-bvo/ [Sourced 2022]
5.
Broaddus, C(2022). Acute Responses to Toxic Exposures. V. in Murray & Nadel’s Textbook of Respiratory Medicine, 2022.
6.
Israel, B (2011). “Brominated Battle: Soda Chemical Has Cloudy Health History.”
Scientific America. 12 December 2011.
7.
Crampton, R., Elias, P., & Gangolli, S. (1971). The bromine content of human tissue. British Journal of Nutrition, 25(2), 317-322.
8.
Munro,IC, Hand,B, Middleton,EJ, Heggtveit,HA, Grice,HC(1972). Toxic effects of brominated vegetable oils in rats, Toxicology and Applied Pharmacology, Volume 22, Issue 3, 1972, Pages 432-439,
9.
Vorhees CV, Butcher RE, Wootten V, Brunner RL(1983). Behavioral and reproductive effects of chronic developmental exposure to brominated vegetable oil in rats. Behavioral Teratology. Volume 28, Issue 3. December 1983
10.
Allain, P., Berre, S., Krari, N., Laine, P., Barbot, N., Rohmer, V., & Bigorgne, J. C. (1993). Bromine and thyroid hormone activity. Journal of clinical pathology, 46(5), 456–458. https://doi.org/10.1136/jcp.46.5.456
11.
Buchberger, W., Holler, W., & Winsauer, K. (1990). Effects of sodium bromide on the biosynthesis of thyroid hormones and brominated/iodinated thyronines. Journal of trace elements and electrolytes in health and disease, 4(1), 25–30.
12.
Kurokawa, Y., Maekawa, A., Takahashi, M., & Hayashi, Y. (1990). Toxicity and Carcinogenicity of Potassium Bromate: A New Renal Carcinogen. Environmental Health Perspectives, 87, 309–335. https://doi.org/10.2307/3431039
13.
Lombardo, Y. B., Chicco, A., Basílico, M. Z., Bernal, C., & Gutman, R. (1985). Effect of brominated vegetable oils on heart lipid metabolism. Lipids, 20(7), 425–432. https://doi.org/10.1007/BF02534233
14.
Orme, J., Taylor, D. H., Laurie, R. D., & Bull, R. J. (1985). Effects of chlorine dioxide on thyroid function in neonatal rats. Journal of toxicology and environmental health, 15(2), 315–322. https://doi.org/10.1080/15287398509530657
15.
Harrington, R. M., Shertzer, H. G., & Bercz, J. P. (1986). Effects of chlorine dioxide on thyroid function in the African green monkey and the rat. Journal of toxicology and environmental health, 19(2), 235–242. https://doi.org/10.1080/15287398609530923
16.
Bercz, J. P., Jones, L. L., Harrington, R. M., Bawa, R., & Condie, L. (1986). Mechanistic aspects of ingested chlorine dioxide on thyroid function: impact of oxidants on iodide metabolism. Environmental health perspectives, 69, 249–254. https://doi.org/10.1289/ehp.8669249
17.
Wones, R. G., Deck, C. C., Stadler, B., Roark, S., Hogg, E., & Frohman, L. A. (1993). Lack of effect of drinking water chlorine on lipid and thyroid metabolism in healthy humans. Environmental health perspectives, 99, 375–381. https://doi.org/10.1289/ehp.9399375
Harrington RM, et al(1986). “Effects of chlorine dioxide on thyroid function in the African green monkey and the rat.” J Toxicol Environ Health. 1986;19(2),235-42.
18.
Epa.gov (2016). Summary: Chloroform. U.S. Environmental Protection Agency [Updated January 2000]. https://www.epa.gov/sites/production/files/2016-09/documents/chloroform.pdf
Li J-H, et al (2015). “Health Effects from Swimming Training in Chlorinated Pools and the Corresponding Metabolic Stress Pathways.” PLoS One. 2015;10(3),e0119241.
19.
EPA.gov (March 8, 2021). Chloramines in Drinking Water. U.S. Environmental Protection Agency. https://www.epa.gov/dwreginfo/chloramines-drinking-water
20.
Goodman, M., & Hays, S. (2008). Asthma and swimming: a meta-analysis. The Journal of asthma : official journal of the Association for the Care of Asthma, 45(8), 639–647. https://doi.org/10.1080/02770900802165980
21.
Thyroid Nation (2014). Bromine Toxicity Destroys Your Thyroid and Metabolism. Published August 12, 2014. [Sourced Jan. 2022] https://thyroidnation.com/bromine-contributor-iodine-deficiency/
22.
Wu Q, Zhang T, Sun H, Kannan K(2010). Perchlorate in tap water, groundwater, surface waters, and bottled water from China and its association with other inorganic anions and with disinfection by-products. Arch Environ Contam Toxicol. 2010 Apr;58(3):543-50.
23.
Sanchez CA, Crump KS, Krieger RI, Khandaker NR, Gibbs JP(2005). Perchlorate and nitrate in leafy vegetables of North America. Environ Sci Technol. 2005 Dec 15;39(24):9391-7.
24. Blount BC, Pirkle JL, Osterloh JD et al(2006). “Urinary Perchlorate and Thyroid Hormone Levels in Adolescent and Adult Men and Women Living in the United States. Environmental Health Perspectives. Vol. 114, No. 12. 2006
25.
Steinmaus C, Pearl M, Kharrazi M, et al(2016). Thyroid Hormones and Moderate Exposure to Perchlorate during Pregnancy in Women in Southern California. Environ Health Perspect. 2016;124(6):861-867. doi:10.1289/ehp.1409614
26.
Mervish N, Blount B, Valentin-Blasini L, Brenner B, Galvez MP, Wolff MS et al(2012). Temporal variability in urinary concentrations of perchlorate, nitrate, thiocyanate, and iodide among children. J Expo Sci Environ Epidemiol 2012; 22: 212–218.
27.
Huber DR, Blount BC, Mage DT, Letkiewicz FJ, Kumar A, Allen RH(2011). Estimating perchlorate exposure from food and tap water based on US biomonitoring and occurrence data. J Expo Sci Environ Epidemiol 2011; 21: 395–407.
28.
Trumpolt. CW. Crain.M(2005). Perchlorate: Sources, Uses, and Occurrences in the Environment. December 2005. Remediation Journal 16(1):65 – 89
29.
C. Steinmaus, M. Pearl(2016). Thyroid Hormones and Moderate Exposure to Perchlorate during Pregnancy in Women in Southern California. Environmental Health Perspectives. Vol. 124, No. 6 Children’s Health Open Access. https://doi.org/10.1289/ehp.1409614
30.
Taylor P. Okosiene OE(2014). Maternal Perchlorate Levels in Women With Borderline Thyroid Function During Pregnancy and the Cognitive Development of Their Offspring: Data From the Controlled Antenatal Thyroid Study. The Journal of Clinical Endocrinology and Metabolism 99(11):jc20141901. July 2014. DOI:10.1210/jc.2014-1901
31.
Cdc.gov (2013).Perchlorate, Nitrate & Thiocyanate – Urine (PERNT_G). National Health and Nutrition Examination Survey. 2011-2012 Data Documentation, Codebook, and Frequencies. First Published: December 2013.Last Revised: October 2014
32.
Leung AM, Pearce EN, Braverman LE(2010). Perchlorate, iodine and the thyroid. Best Pract Res Clin Endocrinol Metab. 2010;24(1):133-141. doi:10.1016/j.beem.2009.08.009
33.
Li JH, Wang ZH, Zhu XJ, et al(2015). Health effects from swimming training in chlorinated pools and the corresponding metabolic stress pathways. PLoS One. 2015;10(3):e0119241. Published 2015 Mar 5. doi:10.1371/journal.pone.0119241
34.
Suh M, Abraham L, Hixon JG, Proctor DM(2014). The effects of perchlorate, nitrate, and thiocyanate on free thyroxine for potentially sensitive subpopulations of the 2001-2002 and 2007-2008 National Health and Nutrition Examination Surveys. J Expo Sci Environ Epidemiol. 2014 Nov;24(6):579-87. doi: 10.1038/jes.2013.67. Epub 2013 Oct 23. PMID: 24149973.
35.
Corey, LM, Bell, GP & Pleus, RC(2017). Exposure of the US Population to Nitrate, Thiocyanate, Perchlorate, and Iodine Based on NHANES 2005–2014. Bull Environ Contam Toxicol 99, 83–88 (2017). https://doi.org/10.1007/s00128-017-2077-7
36.
EPA.gov(January 2014). Technical Fact Sheet – Perchlorate https://www.epa.gov/sites/default/files/2014-03/documents/ffrrofactsheet_contaminant_perchlorate_january2014_final.pdf
37.
Wolff J (1964). Transport of iodide and other anions in the thyroid gland. Physiol Rev 44:45-90
38.
Blount, BC, Rich, DQ, Valentin-Blasini, L, Lashley, S, Ananth, CV, Murphy, E, Smulian, JC, Spain, BJ, Barr, DB, Ledoux, T, Hore, P, & Robson, M(2009). Perinatal exposure to perchlorate. thiocyanate, and nitrate in New Jersey mothers and newborns. Environmental science & technology, 43(19), 7543–7549. https://doi.org/10.1021/es9008486
39.
Blount BC, Rich DQ, Valentin-Blasini L, et al(2009). Perinatal exposure to perchlorate. thiocyanate, and nitrate in New Jersey mothers and newborns. Environ Sci Technol. 2009;43(19):7543-7549. doi:10.1021/es9008486
40.
Kang Y, Park J, Youn K(2019). Association between urinary phthalate metabolites and obesity in adult Korean population: Korean National Environmental Health Survey (KoNEHS), 2012-2014. Ann Occup Environ Med. 2019 Sep 9;31:e23. doi: 10.35371/aoem.2019.31.e23. PMID: 31620300; PMCID: PMC6779927.
41.
Tonacchera M, Pinchera A, Dimida A, Ferrarini E, Agretti P, Vitti P, Santini F, Crump K, Gibbs J (2004) Relative potencies and additivity of perchlorate, thiocyanate, nitrate and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid 14(12):1012–1019
42.
nidcr.nih.gov (2018).The Story of Fluoridation. National Institute of Dental and craniofacial research. https://www.nidcr.nih.gov/health-info/fluoride/the-story-of-fluoridation [Sourced Jan 2022]
43.
Kanduti, D., Sterbenk, P., & Artnik, B. (2016). FLUORIDE: A REVIEW OF USE AND EFFECTS ON HEALTH. Materia socio-medica, 28(2), 133–137. https://doi.org/10.5455/msm.2016.28.133-137
DenBesten, P., & Li, W. (2011). Chronic fluoride toxicity: dental fluorosis. Monographs in oral science, 22, 81–96. https://doi.org/10.1159/000327028
44.
Mouthhealthy.org(2016). Fluorosis. American Dental Association https://www.mouthhealthy.org/en/az-topics/f/fluorosis
Peckham S, Awofeso N. “Water Fluoridation: A Critical Review of the Physiological Effects of Ingested Fluoride as a Public Health Intervention.” ScientificWorldJournal. 2014; 2014,293019.
45.
Connett, P(2022). Search: Fluoride. Fluoride Action Network. https://fluoridealert.org/search-results/?q=fluoride
46.
Błażewicz A, Wiśniewska P, Skórzyńska-Dziduszko K(2021). Selected Essential and Toxic Chemical Elements in Hypothyroidism-A Literature Review (2001-2021). Int J Mol Sci. 2021;22(18):10147. Published 2021 Sep 20. doi:10.3390/ijms221810147
47.
Singh, N., Verma, K. G., Verma, P., Sidhu, G. K., & Sachdeva, S. (2014). A comparative study of fluoride ingestion levels, serum thyroid hormone & TSH level derangements, dental fluorosis status among school children from endemic and non-endemic fluorosis areas. SpringerPlus, 3, 7. https://doi.org/10.1186/2193-1801-3-7
48.
Hopkinsmedicine.org(2022). Hyperparathyroidism. John Hopkins Medicine. https://www.hopkinsmedicine.org/health/conditions-and-diseases/hyperparathyroidism
49.
Czerwinski E, Nowak J, Dabrowska D, Skolarczyk A, Kita B, Ksiezyk M(1988). Bone and joint pathology in fluoride-exposed workers. Arch Environ Health. 1988 Sep-Oct;43(5):340-3.
50.
Chachra D, Limeback H, Willett TL, Grynpas MD(2010). The long-term effects of water fluoridation on the human skeleton. J Dent Res. 2010 Nov;89(11):1219-23. doi: 10.1177/0022034510376070. Epub 2010 Sep 21.
Paiste M, Levine M, Bono JV(2012). Total knee arthroplasty in a patient with skeletal fluorosis. Orthopedics. 2012 Nov;35(11):e1664-7. doi: 10.3928/01477447-20121023-29.
51.
Kanduti D, Sterbenk P, Artnik B(2016). FLUORIDE: A REVIEW OF USE AND EFFECTS ON HEALTH. Mater Sociomed. 2016;28(2):133-137. doi:10.5455/msm.2016.28.133-137
52.
Wikipedia(2022). Fluoridation by country https://en.wikipedia.org/wiki/Fluoridation_by_country
53.
Bánóczy J, Rugg-Gunn A, Woodward M(2013). Milk fluoridation for the prevention of dental caries. Acta Med Acad. 2013 Nov;42(2):156-67. doi: 10.5644/ama2006-124.83. PMID: 24308395.
54.
Marthaler TM(2013). Salt fluoridation and oral health. Acta Med Acad. 2013 Nov;42(2):140-55. doi: 10.5644/ama2006-124.82. PMID: 24308394.
55.
Roschger P, Fratzl P, Schreiber S, Kalchhauser G, Plenk H, Koller K, Eschberggrill J, Klaushofer K(1995). Bone mineral structure after six years fluoride treatment investigated by backscattered electron imaging (BSEI) and small angle x-ray scattering (SAXS): a case report. Bone (Impact Factor: 3.82). 01/1995; 16(3):407-407. DOI:10.1016/8756-3282(95)90480-8.
56.
Wentz, I (2020). Fluoride And Your Thyroid. https://thyroidpharmacist.com/articles/fluoride-and-your-thyroid/
57.
Zeng Q, Cui YS, Zhang L, Fu G, Hou CC, Zhao L, Wang AG, Liu HL(2012). Studies of fluoride on the thyroid cell apoptosis and mechanism. Zhonghua Yu Fang Yi Xue Za Zhi. 2012 Mar;46(3):233-6.
58.
Merck & Co(1968). The Merck Index, 1968 Edition. Rahway NJ. USA. (last accessed 01-10-2014)
59.
Kheradpisheh Z, Mirzaei M, Mahvi AH, et al(2018). Impact of Drinking Water Fluoride on Human Thyroid Hormones: A Case- Control Study. Sci Rep. 2018;8(1):2674. Published 2018 Feb 8. doi:10.1038/s41598-018-20696-4
60.
Peckham S, Lowery D, Spencer S(2015). Are fluoride levels in drinking water associated with hypothyroidism prevalence in England? A large observational study of GP practice data and fluoride levels in drinking water. Journal of Epidemiology and Community Health. 2015;69(7):619-624. doi:10.1136/jech-2014-204971.
61.
Mullenix, P. J., Denbesten, P. K., Schunior, A., & Kernan, W. J. (1995). Neurotoxicity of sodium fluoride in rats. Neurotoxicology and teratology, 17(2), 169–177. https://doi.org/10.1016/0892-0362(94)00070-t
62.
Ravera S, Reyna-Neyra A, Ferrandino G, Amzel LM, Carrasco N(2017). The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu Rev Physiol. 2017;79:261-289. doi:10.1146/annurev-physiol-022516-034125
63.
Liu F, Ma J, Zhang H, Liu P, Liu YP, Xing B, et al(2014). Fluoride exposure during development affects both cognition and emotion in mice. Physiol Behav 124:1–7, PMID: 24184405, doi:10.1016/j.physbeh.2013.10.027.
64.
Choi, A. L., Sun, G., Zhang, Y., & Grandjean, P. (2012). Developmental fluoride neurotoxicity: a systematic review and meta-analysis. Environmental health perspectives, 120(10), 1362–1368. https://doi.org/10.1289/ehp.1104912
65.
Grandjean P, Herz KT(2011). Methylmercury and brain development: imprecision and underestimation of developmental neurotoxicity in humans. Mt Sinai J Med78(1):107–118, PMID: 21259267, doi:10.1002/msj.20228.
66.
Bashash, M., Thomas, D., Hu, H., Martinez-Mier, E. A., Sanchez, B. N., Basu, N., Peterson, K. E., Ettinger, A. S., Wright, R., Zhang, Z., Liu, Y., Schnaas, L., Mercado-García, A., Téllez-Rojo, M. M., & Hernández-Avila, M. (2017). Prenatal Fluoride Exposure and Cognitive Outcomes in Children at 4 and 6-12 Years of Age in Mexico. Environmental health perspectives, 125(9), 097017. https://doi.org/10.1289/EHP655
67.
Tang QQ, Du J(2008). Fluoride and children’s intelligence: a meta-analysis. Biol Trace Elem Res. 2008;126(1-3):115-20. Epub 2008 Aug 10.
68.
Green R, Lanphear B, Hornung R, et al(2019). Association Between Maternal Fluoride Exposure During Pregnancy and IQ Scores in Offspring in Canada. JAMA Pediatr. 2019;173(10):940–948. doi:10.1001/jamapediatrics.2019.1729
69.
Ren, Li, Liu(2008) .A study of the intellectual ability of 8–14 year-old children in high fluoride, low iodine areas. Translated research note with the permission of the Chinese Journal of Control of Endemic Diseases. Fluoride 41(4)319–320 October-December 2008
70.
Wang J, Ge Y, et al(2004). Effects Of High Fluoride And Low Iodine On Biochemical Indexes Of The Brain And Learning-Memory Of Offspring Rats. Fluoride 2004;37(3):201-8 Research report 201
71.
Freni SC(1994). Exposure to high fluoride concentrations in drinking water is associated with decreased birth rates. J Toxicol Environ Health. 1994 May;42(1):109-21.
72.
Zhou Y, Qiu Y, He J, Chen X, Ding Y, Wang Y, Liu X(2013). The toxicity mechanism of sodium fluoride on fertility in female rats. Food Chem Toxicol. 2013 Dec;62:566-72. doi: 10.1016/j.fct.2013.09.023. Epub 2013 Sep 23.
73.
Grzegorzewska AK, Grot E, Sechman A(2021). Sodium Fluoride In Vitro Treatment Affects the Expression of Gonadotropin and Steroid Hormone Receptors in Chicken Embryonic Gonads. Animals (Basel). 2021;11(4):943. Published 2021 Mar 26. doi:10.3390/ani11040943
74.
Luke J(1997). The Effect of Fluoride on the Physiology of the Pineal Gland. Ph.D Dissertation, School of Biological Sciences, University of Surrey, UK. 1997.
Luke J(2001). Fluoride deposition in the aged human pineal gland. Caries Res. 2001 Mar-Apr;35(2):125-8.