 We have all probably heard mitochondria being referred to as “the powerhouse” of our cells and that they play a large role in providing us with most of the energy we need to go about our daily lives. However, many of us, are likely to be unaware of the vast number of other important functions our mitochondria perform and the major role they have on our overall health and the aging process itself.
We have all probably heard mitochondria being referred to as “the powerhouse” of our cells and that they play a large role in providing us with most of the energy we need to go about our daily lives. However, many of us, are likely to be unaware of the vast number of other important functions our mitochondria perform and the major role they have on our overall health and the aging process itself.
What are Mitochondria?
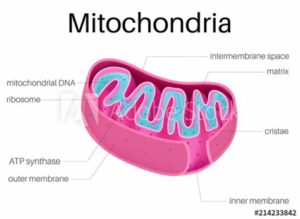
Mitochondria are membrane-bound organelles present in almost all our cells. Responsible for orchestrating cellular energy production, they are central to the maintenance of life and the gatekeepers of cell death.2 Cells such as muscle cells, require much higher densities of mitochondria due to their higher energy requirements whilst other cells require very few mitochondria or none at all, such as our red blood cells.
Mitochondrial Functions
Mitochondria produce about 90 percent of the energy being generated in our bodies in the form of Adenosine Triphosphate (ATP). What many fail to realise is that absolutely everything that happens in our body, every heartbeat, nerve impulse, the breath of air, and so on, requires energy. And because the mitochondria are so critical to that energy supply, any time you have a decrease in that energy production, things can start to fall apart.
A large amount of adenosine triphosphate (ATP) must be produced by the mitochondria every second of each individual day because; ATP cannot be stored. A healthy person at rest produces their body weight in adenosine triphosphate (the equivalent of 1200 watts) every day!3 The brain for example uses 70% of our ATP, this helps explain the strong correlation between mitochondrial dysfunction and neurodegeneration.3
Mitochondria also have other radically important functions. For example, they act as a coordinator for apoptosis, or programmed cell death — an important process that ensures the death of malfunctioning cells that might otherwise develop into tumours. Apoptosis is basically cell suicide. Over the course of a cell’s life, it progressively becomes more and more damaged. When that damage crosses a threshold, mitochondria release cytochrome C, which activates caspases, one of the chief enzymes involved in destroying cells during apoptosis. In this way mitochondria play a role in telling the cell, ’You’re no longer functional, you need to commit cell suicide for the greater good of the organism’. Due to this process, dysfunctional mitochondria are thought to play a role in certain diseases such as cancer.
On top of all of this, mitochondria are able to transiently store calcium contributing to calcium homeostasis, and interestingly if we are completely geeking out here for a moment – mitochondria in our brown adipose tissue, have an alternative function of heat production.4 So, to say the health of your mitochondria plays a role in your overall health, would be a significant understatement.
 Mitochondria’s Role in Oxidative Stress
Mitochondria’s Role in Oxidative Stress
The problem with mitochondria is that they are very sensitive to damage from oxidative stress.
The term “oxidative stress” is frequently mentioned in the realm of science but it is not often clear what it means for us or our health. Research indicates that oxidative stress is an underlying cause of many health challenges and diseases including everything from base level inflammation to cardiovascular and kidney disease. Interestingly it also plays a role in miscarriage and complication in pregnancy.
At its most basic level, oxidation is the loss of electrons. When an atom or compound is oxidized, its properties change. For example, unoxidized iron is a strong, structurally sound metal, while oxidized iron is a brittle, reddish powder. To read more about oxidative stress visit our blog…
How does oxidative stress relate to mitochondria?
This process occurs constantly in our environment and naturally within our bodies. Free radicals and other Reactive Oxygen Species (ROS) are derived either from normal essential metabolic processes in the human body (such as the metabolism of oxygen within our mitochondria), or from external sources such as exposure to X-rays, ozone, cigarette smoking, air pollutants, and industrial chemicals (toxins in general) infections, allergens, stress or a diet containing excess calories and/or poor quality food.
These compounds possess one or more unpaired electrons and as such, are rendered highly reactive, often conducting unwanted oxidation reactions in a variety of cell and tissue sites.
Oxidative stress happens when the number of free radicals exceeds the number of antioxidants. That’s when oxidation damages our cells, proteins, and our DNA (genes).
It is important to understand that free radicals are not universally toxic and to understand this further I would recommend reading our article on Oxidative Stress here.… This is because we need some degree of oxidative stress, as they serve useful and beneficial roles with important signaling functions. That said, most of the superoxide radicals formed at the level of the mitochondria are harmful, which is why we need to minimize free radicals as much as possible.
At high enough concentrations ROS can activate mitochondrial apoptotic machinery which ultimately leads to cell death. However, if maintained at low enough concentrations ROS can serve as important signaling molecules. Various regulatory mechanisms converge upon mitochondria to modulate ATP synthesis and ROS production.1 Not to toot their own horn but this does mean mitochondria are kind of a big deal!
Cellular damage is essentially occurring around the clock, and your body has built-in repair mechanisms to continually address this damage. Compared to the DNA in your cell’s nucleus, the DNA in your mitochondria are far more susceptible to damage because it is located in the same area where free radicals are being constantly produced and possess far less protective and repair mechanisms.5
 Symptoms of Impaired Mitochondrial Function and
Symptoms of Impaired Mitochondrial Function and
Associated Pathologies
Because mitochondria are so essential to many bodily functions, even the slightest impairment to their functioning can have detrimental impacts on our health and can often result in other forms of chronic disease. Some of these include Heart Disease, Alzheimer’s, Cancer, and Chronic Fatigue. Whilst fatigue isn’t as life-threatening as cancer, it can be a painful and persistent problem with seemingly no solution. Poor nutrition, prolonged use of certain medications, antibiotic use, and other factors can diminish ATP production and leave our cells without the energy they need to function and peak performance.
Practical Strategies to Optimise Mitochondrial Function
Thanks to living in a toxic environment, feeding our body inappropriate fuel, eating at the wrong time, and not exercising enough: most people have less than optimized mitochondria. If you’ve got low energy levels, it likely that your mitochondria have become weak, damaged, dysfunctional, and switched off over time. If you’d like more energy, here’s what you need to know…
Supporting your mitochondrial health is the single most important thing you can do to help overcome fatigue and increase your energy levels.
The good news is there are many ways to improve your mitochondrial function. The best options ways to optimise mitochondrial function and the most researched, are exercise, calorie restriction, and appropriate nutrition.
 Exercise:
Exercise:
The reason we end up with benefits to the mitochondria via exercise is that when we are physically active, we place an increased energy demand on our cells.
In response … free radicals signal that you need more mitochondria.
So, our body adapts to physical activity by the mitochondria dividing and becoming more efficient. The next time we do some physical activity, it’s less strenuous. We have a greater capacity to generate the energy needed to meet that demand. That also means that the workload of whatever the cell needs to do at rest is now shared amongst a greater number of mitochondria. Think rest and healing.
Each mitochondrion is now under considerably less stress, and therefore generating far fewer free radicals. That’s one of the reasons why physically fit individuals have a lower risk of pretty much all degenerative diseases, including cancer, as well as a longer life span.5
Caloric Restriction:
Calorie restriction (CR) is said to be one of the most robust, nongenetic intervention that increases lifespan and reduces the rate of aging in a variety of species.
CR reduces oxidative stress while it stimulates the proliferation of mitochondria.
Moreover, mitochondria under CR conditions show less oxygen consumption, reduce membrane potential, and generate less reactive oxygen species than controls, but remarkably they can maintain their critical ATP production. In effect, CR can induce an increase in mitochondria capable of efficient and balanced bioenergetics to reduce oxidative stress and also to reduce age-dependent oxidative damage8.
 Intermittent Fasting:
Intermittent Fasting:
Intermittent fasting has a host of pretty incredible health benefits and therapeutic purposes. One of those is keeping the cells youthful, vigorous, and healthy overall. Over time – just like everything that ages – our mtDNA (mitochondrial DNA) take a few hits and suffers some damage. We know that calorie restriction in and of itself has benefits, and one of those is reversing cell damage.
The only problem? We can’t run a major calorie deficit forever.
Intermittent fasting helps to create a natural calorie deficit – in a healthy way, for prolonged periods of time or to mimic the positive effects that calorie restriction has on cellular health by restricting the eating window. Our bodies are constantly hard at work. When we ingest food, our bodies have a big job to do as we process, absorb, and digest the matter. When we’re not taking in food, our bodies are able to relax and spend time repairing.
By giving some of these key bodily functions a rest, we can expect them to be more efficient (just like we are after the weekend). Intermittent fasting and/or calorie restriction has been linked to lessened mtDNA damage and oxidative stress, making intermittent fasting a potent antioxidant… just like many of the foods mentioned in our next blog , Foods And Supplements To Boost Your Mitochondria.
Moreover, the production of free radicals (the things antioxidants fight) is reduced.
Intermittent fasting and caloric restriction have been studied extensively, and it’s often a piece of the puzzle in longevity, which inherently means it’s good for fighting the disease which always begins in our cells. Thus, we’re led to believe it’s advantageous to our cells, too. Intermittent fasting is ideal to do before going to bed when we’re burning the least number of calories (at rest), and into the following morning. Yes – sleep counts as fasting, so you hardly have to try! By eliminating the number of hours during the day we are taking food in, we also naturally eat less.
 The Mitochondrial Theory of Aging.
The Mitochondrial Theory of Aging.
Virtually all the energy that powers the trillions of cells in our body comes from our mitochondria. Here’s the problem: As we age, our mitochondria become damaged and dysfunctional. And they even shrink in size. Think of how a muscle shrinks or atrophies if it’s in a cast — the same thing happens to our mitochondria, our energy generators over time.
Research has shown that for most people, from the age of 20-40, their mitochondrial capacity (the ability of our cells to produce energy) decreases by HALF. And then, from 40-70, it decreases by another HALF! As a result, the energy your mitochondria produce naturally decreases over time, reducing your health and longevity — and ultimately, causing fatigue.
The Mitochondrial Theory of Aging, (a variant of the free radical theory of aging) just because we are nerding out here, proposes that accumulation of damage to the mitochondria and mitochondrial DNA (mtDNA) via the process described early, leads to aging of humans and animals.Remember we said that the production of ATP (energy) and reactive oxygen species (ROS) are intimately linked to the electron transport chain (ETC) within the mitochondria. Electrons from nutrients are passed through the ETC until they reach the terminal electron acceptor, molecular oxygen (O2), which ultimately drives the synthesis of ATP. This process of electron transfer to produce ATP however also results in the production of ROS.Cellular damage is essentially occurring around the clock, and our body has built-in repair mechanisms to continually address this damage. Compared to the DNA in our cell’s nucleus, the DNA in our mitochondria are far more susceptible to damage because it is located in the same area where free radicals are being constantly produced and possess far less protective and repair mechanisms.5
Meaning that Human mtDNA, which is not protected by histones and yet is exposed to high levels of ROS and free radicals in the matrix of mitochondria, is susceptible to oxidative damage and mutation in tissue cells. The incidence and abundance of these mutant mtDNAs are increased with age, particularly in tissues with great demand for energy. Age-related impairment in the mitochondrial enzymes not only decreases ATP synthesis but also enhances production of reactive oxygen species (ROS) through increased electron leakage in the respiratory chain.6
What does this mean?
Well put simply many scientists believe that damage to our mitochondria over time due to oxidative stress and mutations in mitochondrial DNA, is one of the key factors in our aging process. This can be observed in our tissues that require the most amount of energy deteriorating at a faster rate. For example, the retina in our eye has the greatest metabolic demand and highest mitochondrial density in the body and displays progressive age-related inflammation and marked cell loss. We all know that our vision declines as we age. We also know however that those with diabetes tend to have more vision problems than those without diabetes. Also smokers, those who drink excessive amounts of alcohol or those who suffer with chronic disease, also struggle with weaker vision.7
As we’ve discussed mitochondria are organelles that are found inside every living cell, which is why mitochondrial dysfunction can have serious implications for our health. For more details on what foods are imperative for our mitochondria and which supplements assist these energy centres please see our blog, Foods And Supplements To Boost Your Mitochondria.
References:
1. Mailloux RJ, Jin X, Willmore WG. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol. 2013;2:123-139. Published 2013 Dec 19. doi:10.1016/j.redox.2013.12.011
2. Osellame LD, Blacker TS, Duchen MR. Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab. 2012;26(6):711-723. doi:10.1016/j.beem.2012.05.003
3. Pizzorno J. Mitochondria-Fundamental to Life and Health. Integr Med (Encinitas). 2014;13(2):8-15.
4. https://teachmephysiology.com/basics/cell-structures/mitochondria/
5. https://articles.mercola.com/sites/articles/archive/2018/02/25/mitochondria-facts.aspx
6. Wei YH, Ma YS, Lee HC, Lee CF, Lu CY. Mitochondrial theory of aging matures–roles of mtDNA mutation and oxidative stress in human aging. Zhonghua Yi Xue Za Zhi (Taipei). 2001;64(5):259-270.
7. Mitochondrial Dysfunction: A Quick test (art 2)
8. López-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R(2006). Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proceedings of the National Academy of Sciences Feb 2006, 103 (6) 1768-1773; DOI: 10.1073/pnas.0510452103